Sorption of C2H6/CO2 gas mixtures in amorphous glassy polyphenylene oxide: Modelling of thermodynamics and of the multicomponent diffusion process
IF 9
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
This contribution addresses the experimental characterization and the theoretical interpretation of the sorption thermodynamics and diffusion kinetics of a binary gas mixture in a glassy amorphous polymer. Specifically, the multicomponent diffusion of C2H6/CO2 mixtures with a mole fraction of CO2 mol mol−1 has been investigated in a film of Poly(2,6-dimethyl-1,4-phenylene)oxide (PPO) at 308.15 K and at several values of sub-atmospheric pressure. Static experiments were conducted in a closed volume, coupling FTIR spectroscopy in the transmission mode and barometry. Competitive sorption produces a decrease of the equilibrium concentration of each component of the mixture in PPO with respect to the corresponding cases of pure gas sorption performed at pressure values equal to the partial pressure values in the gas mixture. Moreover, during multicomponent diffusion, carbon dioxide attains concentration values exceeding the final sorption equilibrium value, displaying an overshoot in the sorption kinetics curve. To interpret these experimental findings, the glassy polymer phase has been modelled with a lattice fluid model according to the Non-Equilibrium Thermodynamics of Glassy Polymers (NET-GP) theory and, for each penetrant, its chemical potential gradient has been considered as the driving force for diffusion. Theoretical analysis well predicts the competitive sorption thermodynamics and the supra-equilibrium loading of carbon dioxide during multicomponent diffusion. Notably, the model shows that osmotic, reverse and barrier diffusion of carbon dioxide occur at short times prior to the overshoot. Implications of the theoretical analysis on separation performances of a PPO based membranes have also been addressed.

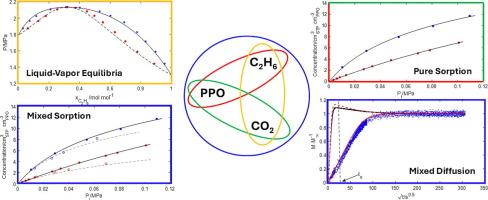
C2H6/CO2气体混合物在非晶态玻璃聚苯氧化物中的吸附:热力学和多组分扩散过程的建模
这一贡献解决了实验表征和理论解释的吸附热力学和扩散动力学的二元气体混合物在玻璃非晶聚合物。具体地说,在308.15 K和几个大气压值下,研究了CO2摩尔分数为0.525 × 0.525 mol mol−1的C2H6/CO2混合物在聚(2,6-二甲基-1,4-苯基)氧化物(PPO)薄膜中的多组分扩散。静态实验在封闭的体积内进行,耦合FTIR光谱在透射模式和气压测量。相对于在等于气体混合物分压值的压力值下进行的纯气体吸附的相应情况,竞争性吸附产生PPO中混合物各组分的平衡浓度的降低。此外,在多组分扩散过程中,二氧化碳的浓化值超过了最终的吸附平衡值,在吸附动力学曲线上表现出超调。为了解释这些实验结果,根据玻璃聚合物的非平衡热力学(NET-GP)理论,用晶格流体模型对玻璃聚合物相进行了建模,并且对于每种渗透剂,其化学势梯度被认为是扩散的驱动力。理论分析很好地预测了多组分扩散过程中二氧化碳的竞争吸附热力学和超平衡负载。值得注意的是,该模型表明,二氧化碳的渗透扩散、反向扩散和屏障扩散在超调之前的短时间内发生。理论分析的意义上的分离性能的PPO基膜也已解决。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


