Electrochemical mechanism and surface phase composition of arsenopyrite oxidation in Cu(II)-ammonia-thiosulfate systems
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
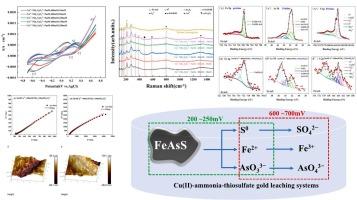
Thiosulfate gold extraction is considered to be the most promising green gold extraction technology for industrial applications. However, there are several long-term problems associated with gold extraction by thiosulfate, including high thiosulfate consumption and gold passivation. Arsenopyrite (FeAsS) is a common associated mineral in gold ores. On the one hand, this mineral encapsulates gold, which is unfavourable for leaching, and on the other hand, arsenic released during gold leaching or ore pretreatment will pollute the environment. Therefore, it is necessary to study the oxidative dissolution behaviour of arsenopyrite in Cu(II)-ammonium thiosulfate gold leaching system. In this study, the electrochemical oxidation behaviour of arsenopyrite in Cu2+-NH3-S2O32− gold leaching systems with different thiosulfate concentrations was investigated by electrochemical techniques and solid-state characterisation. The results show that the initial oxidative dissolution of FeAsS generates AsO33−, Fe2+ and S0, and then the further oxidation generates Fe3+, SO42− and AsO43−; and it is pointed out that the S2O32− concentration in the leaching system that is more capable of promoting the oxidation of arsenopyrite is 100 mM and 150 mM.; elucidated the morphological changes of iron, arsenic and sulphur species on the surface of arsenopyrite before and after oxidation. This study provides a basis for reducing the influence of associated arsenopyrite on thiosulphate gold leaching, and has certain practical significance for promoting the industrialisation of thiosulphate gold leaching.

铜(II)-氨-硫代硫酸盐体系中毒砂氧化的电化学机理和表面相组成
硫代硫酸盐提金被认为是工业应用中最有前途的绿色提金技术。然而,硫代硫酸盐提金存在几个长期问题,包括硫代硫酸盐消耗量高和金钝化。黄铜矿(FeAsS)是金矿石中常见的伴生矿物。一方面,这种矿物会包裹金,不利于浸出;另一方面,金浸出或矿石预处理过程中释放的砷会污染环境。因此,有必要研究黄铜矿在铜(II)-硫代硫酸铵金浸出体系中的氧化溶解行为。本研究采用电化学技术和固态表征方法,研究了不同硫代硫酸盐浓度的 Cu2+-NH3-S2O32- 黄金浸出体系中黄砷矿的电化学氧化行为。结果表明,FeAsS 初始氧化溶解生成 AsO33-、Fe2+ 和 S0,进一步氧化生成 Fe3+、SO42- 和 AsO43-,并指出浸金体系中更能促进砷黄铁矿氧化的 S2O32- 浓度分别为 100 mM 和 150 mM;阐明了氧化前后砷黄铁矿表面铁、砷、硫物种的形态变化。该研究为降低伴生黄砷矿对硫代硫酸浸金的影响提供了依据,对促进硫代硫酸浸金工业化具有一定的现实意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


