A Sm(II)-based catalyst for the reduction of dinitrogen, nitrite, and nitrate to ammonia or urea
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Industrial dinitrogen (N2) reduction to ammonia in the Haber-Bosch synthesis is essential for producing fertilizers and, consequently, food. Methods wherein the energy for nitrogen activation is supplied by light could provide more sustainable alternatives to existing ones. The combination of a photosensitizer and a lanthanide catalyst is reported for an effective >2e− reduction of N2 in what is the first transition-metal-free molecular photocatalyst for ammonia synthesis. The lanthanide is Earth-abundant Sm. The reaction proceeds at ambient pressure and temperature, with high turnover numbers (up to 98), with visible light irradiation in aqueous solvent mixtures and even pure water, and it uses an environmentally benign non-metallic sacrificial reductant. Nitrite and nitrate were also efficiently reduced to ammonia. Thus, the first photocatalytic co-reduction of nitrite and bicarbonate to urea using an Sm-based photocatalyst was achieved.

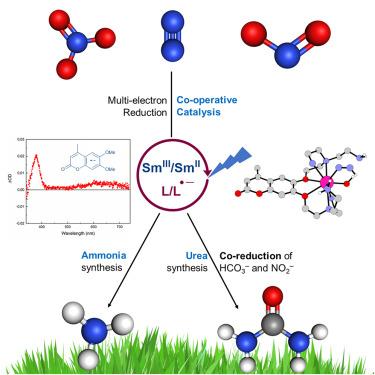
一种Sm(II)基催化剂,用于将二氮、亚硝酸盐和硝酸盐还原为氨或尿素
在Haber-Bosch合成中,工业二氮(N2)还原为氨是生产肥料和食品所必需的。由光提供氮活化能量的方法可以为现有方法提供更可持续的替代方案。据报道,光敏剂和镧系催化剂的组合有效地还原了N2,这是第一个用于合成氨的无过渡金属分子光催化剂。镧系元素是地球上丰富的钐。该反应在环境压力和温度下进行,周转率高(高达98),在含水溶剂混合物甚至纯水中进行可见光照射,并使用无害环境的非金属牺牲还原剂。亚硝酸盐和硝酸盐也被有效地还原为氨。因此,首次实现了使用sm基光催化剂将亚硝酸盐和碳酸氢盐共还原为尿素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


