Retrobiosynthesis of unnatural lactams via reprogrammed polyketide synthase
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Engineered polyketide synthases (PKSs) have great potential as biocatalysts. These unnatural enzymes are capable of synthesizing molecules that are either not amenable to biosynthesis or are extremely challenging to access chemically. PKSs can thus be a powerful platform to expand the chemical landscape beyond the limits of conventional metabolic engineering. Here we employ a retrobiosynthesis approach to design and construct PKSs to produce δ-valerolactam (VL) and three enantiopure α-substituted VL analogues that have no known biosynthetic route. We introduce the engineered PKSs and pathways for various malonyl-CoA derivatives into Pseudomonas putida and use proteomics, metabolomics and culture condition optimization to improve the production of our target compounds. These α-substituted VLs are polymerized into polyamides (nylon-5) or converted into their N-acryloyl derivatives. RAFT polymerization produces bio-derived polymers with potential biomedical applications. Overall, this interdisciplinary effort highlights the versatility and effectiveness of a PKS-based retrobiosynthesis approach in exploring and developing innovative biomaterials. Engineered polyketide synthases (PKSs) have great potential as biocatalysts for the synthesis of chemically challenging molecules. Here the authors show a retrobiosynthesis approach to design and construct PKSs to produce a series of valerolactams for biopolymer production.
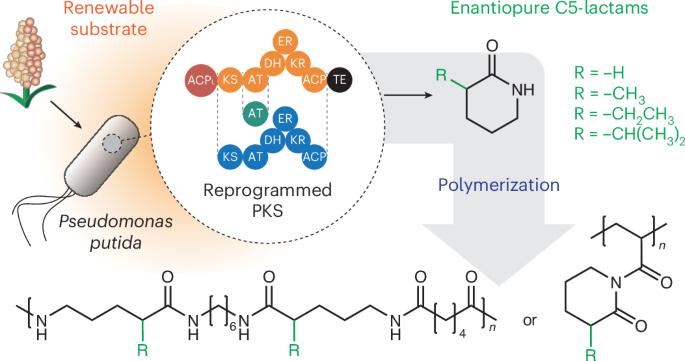

通过重编程聚酮合成酶逆转录合成非天然内酰胺
工程聚酮合成酶(pks)作为生物催化剂具有巨大的潜力。这些非天然酶能够合成一些分子,这些分子要么无法进行生物合成,要么极难通过化学方法获得。因此,pks可以成为一个强大的平台,超越传统代谢工程的限制,扩展化学景观。本研究采用逆转录生物合成方法设计和构建pks,以生产δ-戊内酯(VL)和三种对映纯α-取代的VL类似物,这些类似物没有已知的生物合成途径。我们将工程的pks和各种丙二酰辅酶a衍生物进入恶臭假单胞菌的途径,并使用蛋白质组学,代谢组学和培养条件优化来提高目标化合物的产量。这些α-取代的VLs被聚合成聚酰胺(尼龙-5)或转化成它们的n-丙烯酰衍生物。RAFT聚合生产具有潜在生物医学应用的生物衍生聚合物。总的来说,这一跨学科的努力突出了以pks为基础的反生物合成方法在探索和开发创新生物材料方面的多功能性和有效性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


