The Temperature Dependence of the Langmuir Adsorption Model for a Single-Site Metal–Organic Framework
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
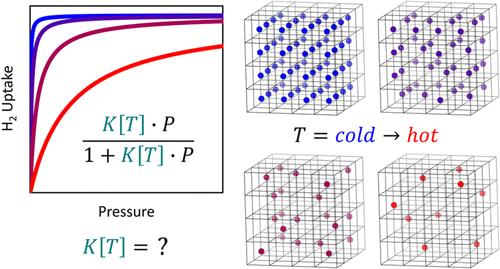
The single-site Langmuir adsorption model, also known as the Langmuir isotherm equation, is one of the simplest possible descriptions of adsorption phenomena and yet finds widespread applicability across a range of disciplines. In its simplest form, it is deployed to treat adsorption equilibria at constant temperature (i.e., along isotherms); however, at the heart of its derivation is a more general class of models that each incorporates an explicit temperature dependence, subject to assumptions about the spatial/translational degrees of freedom of the adsorbed species. In this work, measurements of the temperature dependence of supercritical adsorption of H2 on a single-site metal–organic framework (MOF) are presented and fitted using a range of Langmuir models with distinct treatments of degrees of freedom in the adsorbed phase. Surprisingly, all of the models can be used to adequately represent the measured data (to within 0.0003 mmol g–1 per point), despite yielding significantly different values for binding energy and the temperature dependence of the isosteric enthalpy of adsorption (i.e., the isosteric heat, qst). However, a critical finding of this work is that the mean-temperature isosteric enthalpy of adsorption remains consistent across all models within experimental error (±0.1% or <0.1 kJ mol–1), highlighting its reliability for evaluating adsorption thermodynamics.
单点金属-有机骨架Langmuir吸附模型的温度依赖性
单点Langmuir吸附模型,也被称为Langmuir等温线方程,是对吸附现象最简单的描述之一,但在一系列学科中有着广泛的适用性。在其最简单的形式中,它用于处理恒温(即沿等温线)下的吸附平衡;然而,其推导的核心是一类更一般的模型,每个模型都包含一个明确的温度依赖,受制于对吸附物质的空间/平动自由度的假设。在这项工作中,提出了H2在单位点金属有机框架(MOF)上超临界吸附的温度依赖性的测量,并使用一系列Langmuir模型进行了拟合,这些模型具有不同的吸附相自由度处理。令人惊讶的是,所有的模型都可以用来充分地表示测量数据(在每点0.0003 mmol g-1以内),尽管结合能和等容吸附焓(即等容热,qst)的温度依赖关系的值有很大不同。然而,这项工作的一个关键发现是,在实验误差(±0.1%或<;0.1 kJ mol-1)范围内,所有模型的平均温度等容焓保持一致,突出了其评估吸附热力学的可靠性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


