Elucidation of an Unusually Long Pu–N Bond in a Plutonium(III)-Tetrazolate Complex
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Four trivalent, f-element tetrazolate hydrate complexes [M(H2O)9](Hdtb)3·nH2O (Nd1, n = 7 and Pu1, n = 9; dtb2– = 1,3-di(tetrazolate-5-yl)benzene) and [M(Hdtb)(H2O)8](dtb)·11H2O (Nd2 and Pu2) were prepared using metathesis reactions. These complexes contain hydrated M(III) cations, but in the latter complexes, Nd2 and Pu2, one of the water molecules has been displaced by a long interaction between the M(III) cation and a Hdtb– anion. Notably, the Pu(III)–N bond in Pu2, representing the longest IXPu(III)–N (IX = nine coordinate) bond reported has a length of 2.8338(15) Å and is slightly shorter than the Nd(III)–N bond length of 2.8425(13) Å in Nd2. Analysis of bond lengths, Wiberg bond indices (WBI), natural localized molecular orbitals (NLMOs), and quantum theory of atoms in molecules (QTAIM) reveals that the metal contribution to the Pu(III)–N bond is marginally greater than that of the Pu(III)–OH2 bonds in Pu2 and the Nd(III)–N bond in Nd2. Thus, this rather long M–N interaction provides an example where the expectation that An(III) compounds exhibit greater covalency with soft donor ligands compared to harder ligands fails. The absorption spectra of Pu1 and Pu2 further support this observation, highlighting a surprising degree of similarity in their electronic structures.
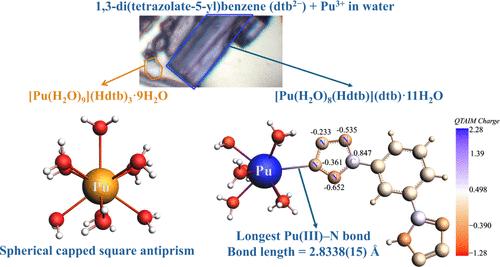
钚(III)-四氮酸盐配合物中异常长Pu-N键的解释
四种三价f元素四氮酸盐水合物配合物[M(H2O)9](Hdtb)3·nH2O (Nd1, n = 7和Pu1, n = 9);通过还原反应制备了[M(Hdtb)(H2O)8](dtb)·11H2O (Nd2和Pu2)。这些配合物含有水合M(III)阳离子,但在后一种配合物中,Nd2和Pu2,其中一个水分子被M(III)阳离子和Hdtb -阴离子之间的长时间相互作用所取代。值得注意的是,Pu2中的Pu(III) -N键代表了报道的最长的IXPu(III) -N (IX = 9坐标)键,其长度为2.8338(15)Å,略短于Nd2中的Nd(III) -N键长度2.8425(13)Å。对键长、Wiberg键指数(WBI)、自然定域分子轨道(NLMOs)和分子原子量子理论(QTAIM)的分析表明,金属对Pu(III) -N键的贡献略大于Pu2中的Pu(III) -OH2键和Nd2中的Nd(III) -N键的贡献。因此,这种相当长的M-N相互作用提供了一个例子,表明与硬配体相比,an (III)化合物与软供体配体表现出更大共价的期望失败了。Pu1和Pu2的吸收光谱进一步支持了这一观察结果,突出了它们电子结构的惊人相似性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


