Practical flow battery diagnostics enabled by chemically mediated monitoring
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aqueous organic flow batteries are a promising technology class for long-duration energy storage. However, the poor stability of redox-active components under the conditions frequently used in these batteries, coupled with the inherently high degree of active material chemical complexity, frequently gives rise to intricate degradation pathways that both limit attainable cycle life and are challenging to probe experimentally. Here, we utilize solution pH and bulk magnetic susceptibility to monitor the native minor equilibrium side reaction between water and the one-electron oxidized state of 2,2,6,6-tetramethyl-4-hydroxy-piperidin-1-oxyl (4-hydroxy-TEMPO)—an archetypical flow battery catholyte. This side reaction readily reports on both the main redox reaction of 4-hydroxy-TEMPO, which itself is not proton coupled, as well as on its principal self-discharge pathway. In so doing, it provides accurate, low-cost, and sensitive experimental insights into battery state of charge, state of health, and operating conditions for both flow and hybrid flow configurations.

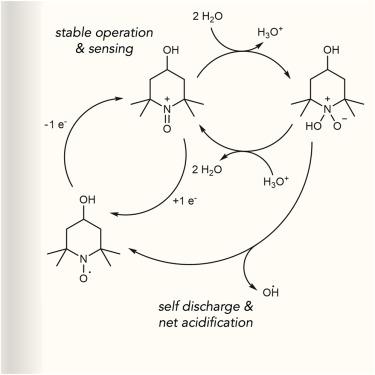
通过化学介导监测实现实用的液流电池诊断技术
水性有机液流电池是一种很有前景的长时间储能技术。然而,在这些电池经常使用的条件下,氧化还原活性成分的稳定性较差,再加上活性材料化学性质本身的高度复杂性,经常会产生错综复杂的降解途径,既限制了可达到的循环寿命,又对实验探测构成了挑战。在这里,我们利用溶液 pH 值和块状磁感应强度来监测水与 2,2,6,6- 四甲基-4-羟基哌啶-1-氧(4-羟基-TEMPO)的单电子氧化态之间的原生次要平衡副反应。这种副反应可随时报告 4-hydroxy-TEMPO 的主要氧化还原反应(其本身并不是质子耦合反应)及其主要自放电途径。这样,它就能为液流电池和混合液流电池配置提供准确、低成本和灵敏的电池充电状态、健康状态和工作条件实验见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


