Hydrothermal enhanced etching of Ni for direct recovery of gold flakes from electronic waste†
IF 9.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The content of precious Au in electronic waste (e-waste) is often tens to hundreds of times higher than that in natural ore deposits. Current technologies for recovering Au from e-waste mainly involve a two-step process of leaching and reduction, which are accompanied by high energy consumption, greenhouse gas emissions and toxic agents. In this article, we present an efficient, environmentally friendly and scalable hydrothermal catalysis technique for the one-step recovery of Au solid flakes from e-waste. The recovery process avoids the use of strong acids, alkalis, or toxic agents and operates under mild conditions (80–130 °C). During the hydrothermal reaction, Ni beneath the Au layer is selectively etched by hydroxyl radicals (˙OH), allowing the Au layer to be directly peeled off and recovered. Au solid flakes with a high recovery rate (up to 99.2%) and high purity (96.6%, without a further purification process) are obtained, eliminating the need for any additional reduction process. This research emphasizes the crucial role of both hydrothermal conditions and catalysts (e.g. TiO2) in promoting the generation of ˙OH. The results of an techno-economic analysis and life cycle assessment indicate that this hydrothermal catalysis technique is a low-cost and environmentally friendly method for large-scale Au recovery from e-waste.
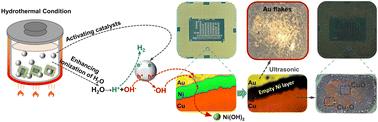
水热强化镍刻蚀法直接回收电子废弃物中金片†
电子废弃物中贵重金的含量往往比天然矿床高出数十至数百倍。目前从电子垃圾中回收金的技术主要涉及浸出和还原两步过程,这伴随着高能耗、温室气体排放和有毒物质。本文介绍了一种高效、环保、可扩展的水热催化技术,用于一步回收电子垃圾中的金固体薄片。回收过程避免使用强酸、强碱或有毒药剂,并在温和的条件下(80-130°C)操作。在水热反应过程中,Au层下方的Ni被羟基自由基(˙OH)选择性蚀刻,使Au层被直接剥离和回收。获得了高回收率(99.2%)和高纯度(96.6%,无需进一步提纯)的金固体薄片,无需任何额外的还原过程。本研究强调了水热条件和催化剂(如TiO2)在促进˙OH生成中的关键作用。技术经济分析和生命周期评价结果表明,水热催化技术是一种低成本、环保的从电子垃圾中大规模回收金的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Green Chemistry
化学-化学综合
CiteScore
16.10
自引率
7.10%
发文量
677
审稿时长
1.4 months
期刊介绍:
Green Chemistry is a journal that provides a unique forum for the publication of innovative research on the development of alternative green and sustainable technologies. The scope of Green Chemistry is based on the definition proposed by Anastas and Warner (Green Chemistry: Theory and Practice, P T Anastas and J C Warner, Oxford University Press, Oxford, 1998), which defines green chemistry as the utilisation of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products. Green Chemistry aims to reduce the environmental impact of the chemical enterprise by developing a technology base that is inherently non-toxic to living things and the environment. The journal welcomes submissions on all aspects of research relating to this endeavor and publishes original and significant cutting-edge research that is likely to be of wide general appeal. For a work to be published, it must present a significant advance in green chemistry, including a comparison with existing methods and a demonstration of advantages over those methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


