The superhydrophilic and superaerophobic properties and electron redistribution synergistically boost cobalt nanoparticle encapsulated N-doped carbon nanotube arrays electrode for the electrocatalytic overall water splitting performance
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The development of efficient and stable transition metals (TMs)-based bifunctional electrocatalysts to enhance the hydrogen and oxygen evolution reactions (HER and OER) are critical yet extremely challenging for improving the energy conversion efficiency of overall water splitting (OWS). Thus, by skillfully constructing three-dimensional (3D) array structure and introducing heteroatom N with similar electronegativity to C, a series of self-supported electrodes composed of N-doped carbon nanotube (NCNT) arrays were synthesized via one-step calcination, in which Co was wrapped around the tip of NCNT (Co@NCNT/NF-x, where x = 50, 100, 150). Studies have demonstrated that the resulting Co@NCNT/NF-100 electrode displays the optimal electroactivities in HER (117 mV at 10 mA cm−2), OER (207 mV at 10 mA cm−2) and OWS (1.57 V at 10 mA cm−2) along with long-term stability exceeding 100 h. The oriented arrangement of NCNT arrays facilitates electrolyte permeation and bubbles escape, imparting superhydrophilic and superaerophobic features to the self-supported electrode. Theoretical simulations reveal that the introduction of N into the carbon skeleton can effectively regulate the electronic structure and optimize the adsorption free energy of key intermediates. This work clearly reveals the structure–activity relationship of Co@NCNT/NF-100 electrode and provides valuable insights for designing more advanced TMs-based bifunctional electrocatalysts.
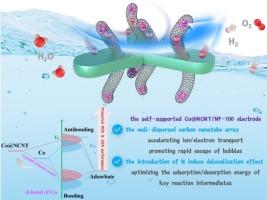
超亲水性和超疏水性以及电子重分布协同提高了钴纳米颗粒包封n掺杂碳纳米管阵列电极的电催化整体水分解性能
开发高效、稳定的过渡金属双功能电催化剂来促进氢和氧的析出反应(HER和OER)是提高整体水分解(OWS)能量转换效率的关键,但也是极具挑战性的。因此,通过巧妙地构建三维(3D)阵列结构,并引入与C电负性相似的杂原子N,通过一步煅烧合成了一系列由N掺杂碳纳米管(NCNT)阵列组成的自支撑电极,其中Co包裹在NCNT的尖端(Co@NCNT/NF-x,其中 x = 50,100,150)。研究已经证明,由此产生的Co@NCNT / nf - 100电极显示最优electroactivities在她的马(117 mV 10 厘米−2),超过10点(207 mV 马 厘米−2)和OWS (1.57 V 10马 厘米−2)以及长期稳定超过100 h。纳米碳纳米管阵列的定向排列有利于电解质渗透和气泡逸出,赋予自支撑电极超亲水性和超疏氧特性。理论模拟结果表明,在碳骨架中引入N可以有效调节电子结构,优化关键中间体的吸附自由能。这项工作清楚地揭示了Co@NCNT/NF-100电极的结构-活性关系,为设计更先进的基于tms的双功能电催化剂提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


