Electron Perturbation for Chiral DNA Point Mutation
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Advances in molecular nanotechnology have enabled the design of systems that exploit nanoscale interactions for enhanced biosensing and diagnostics. Here, we present a plasmonic nematic film (PNF) that leverages nanoscale plasmonic hotspots to amplify electron perturbations induced by DNA mutations. Sequence-specific mismatches, particularly point mutations, significantly alter the local electromagnetic environment, leading to distinct and quantifiable spectral shifts in circular dichroism (CD), denoted as Δλdip. These shifts exhibit a strong correlation with target DNA concentration (R2 > 0.99), enabling precise, quantitative detection of mutation-induced asymmetry. The underlying mechanism is modeled by the asymmetric chiral signal Iasy = ∫ΨPNF*(Ω)ΨPNF dV, where ΨPNF is the wave function of the PNF and Ω represents its chiroptical response. Simulations and electric field analysis further validate that mutation-driven perturbations at the PNF-DNA interface enhance local field intensity at λdip, while no significant changes occur at nonresonant wavelengths. Through this mechanism, the PNF platform achieves over 240% enhancement in chiroptical signal compared to wild-type DNA and enables mutation detection down to 1534 pg. These findings highlight the system’s potential for high-specificity diagnostics of clinically relevant mutations, including those associated with hereditary hearing impairment, and may inform the development of future chiral biosensing platforms.
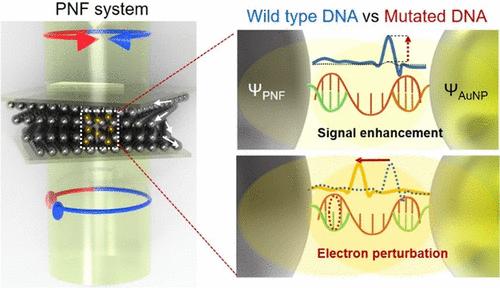
手性DNA点突变的电子扰动
分子纳米技术的进步使得利用纳米级相互作用来增强生物传感和诊断的系统设计成为可能。在这里,我们提出了一种等离子体向列膜(PNF),它利用纳米级等离子体热点来放大DNA突变引起的电子扰动。序列特异性错配,特别是点突变,显著改变了局部电磁环境,导致圆二色性(CD)中明显且可量化的光谱偏移,表示为Δλdip。这些变化与目标DNA浓度有很强的相关性(R2 >;0.99),能够精确、定量地检测突变引起的不对称性。潜在的机制是通过不对称手性信号Iasy =∫ΨPNF*(Ω)ΨPNF dV来建模的,其中ΨPNF是PNF的波函数,Ω表示其手性响应。模拟和电场分析进一步验证了突变驱动的PNF-DNA界面微扰增强了λdip处的局部场强,而在非共振波长处没有显著变化。通过这种机制,与野生型DNA相比,PNF平台实现了超过240%的手性信号增强,并且能够检测到低至1534 pg的突变。这些发现突出了该系统在临床相关突变(包括与遗传性听力障碍相关的突变)的高特异性诊断方面的潜力,并可能为未来手性生物传感平台的发展提供信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


