Surface reconstruction of Cu-Doped Ni(OH)2 during Electrolysis for enhanced urea oxidation
IF 6.9
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In addressing the challenge posed by the substantial overpotential required by oxygen evolution reaction (OER), considerable attention has been directed toward urea oxidation reaction (UOR) as a promising alternative. This oxidation process reduces the energy input commonly linked to the excessive overpotential in OER and concurrently enables the elimination of urea from wastewater streams. These combined attributes indicate considerable promise for practical implementation in industrial contexts. Nickel (Ni)-based hydroxides have been widely recognized for their significant role in UOR. In this study, Cu doping was employed to enhance the valence state of Ni atoms in Ni(OH)2, thereby facilitating the formation of Ni oxyhydroxide (NiOOH). The Cu-Ni(OH)2 catalyst exhibited significantly enhanced catalytic activity in UOR compared to pure Ni(OH)2. The synthesized catalysts exhibited exceptional performance in the urea oxidation reaction, achieving a low operational voltage of 1.283 V at a current density of 50 mA cm−2, while also demonstrating impressive stability over extended periods, exceeding 150 h. The findings of this investigation offer valuable theoretical perspectives for the development of non-precious metal-based catalysts, which can efficiently promote surface reorganization during the reaction.
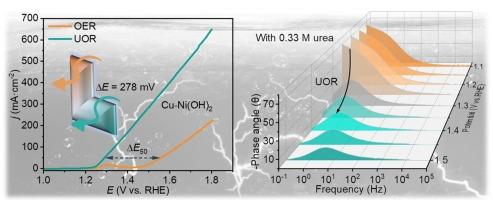
cu掺杂Ni(OH)2在电解强化尿素氧化过程中的表面重构
为了解决析氧反应(OER)所需的大量过电位所带来的挑战,尿素氧化反应(UOR)作为一种有前途的替代方法受到了相当大的关注。这种氧化过程减少了通常与OER中过度过电位相关的能量输入,同时能够从废水流中消除尿素。这些综合属性显示了在工业环境中实际实施的可观前景。镍基氢氧化物因其在UOR中的重要作用而得到广泛认可。本研究采用Cu掺杂的方法增强了Ni(OH)2中Ni原子的价态,从而促进了NiOOH的形成。与纯Ni(OH)2相比,Cu-Ni(OH)2催化剂在UOR中的催化活性显著增强。合成的催化剂在尿素氧化反应中表现出优异的性能,在50 mA cm−2的电流密度下达到1.283 V的低工作电压,同时在超过150 h的长时间内也表现出令人印象深刻的稳定性。本研究结果为开发非贵金属基催化剂提供了有价值的理论视角,这些催化剂可以有效地促进反应过程中的表面重组。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


