Effects of Compression on the Local Iodine Environment in Dipotassium Zinc Tetraiodate(V) Dihydrate K2Zn(IO3)4·2H2O
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Combining X-ray diffraction with density-functional theory and electron topology calculations, we found that pressure substantially modifies the bonding in K2Zn(IO3)4·2H2O. We discovered that under compression, there is a progressive change from primary covalent I–O bonds and secondary halogen I···O interactions toward O–I–O electron-deficient multicenter bonds. Because of this, iodine hypercoordination converts IO3 trigonal pyramids toward IO6 units. The formation of these IO6 units breaks the typical isolation of iodate molecules, forming an infinite two-dimensional iodate network. Hypercoordination influences the hydrogen atoms too, such that multicenter O–H–O bonds are also promoted with increasing pressure. We have determined that K2Zn(IO3)4·2H2O is one of the most compressible iodates studied to date, with a bulk modulus of 22(3) GPa. The pressure-induced structural changes strongly modify the electronic structure as shown by optical-absorption measurements and band-structure calculations. The band gap energy closes from 4.2(1) eV at ambient pressure to 3.4(1) eV at 20 GPa.
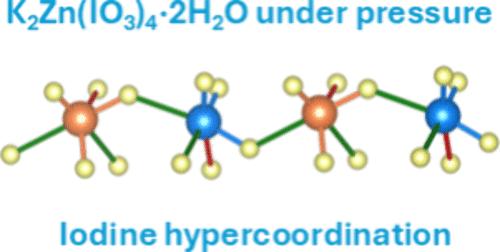
压缩对四碘酸锌(V)二水合物K2Zn(IO3)4·2H2O局部碘环境的影响
通过将 X 射线衍射与密度泛函理论和电子拓扑计算相结合,我们发现压力极大地改变了 K2Zn(IO3)4-2H2O 中的键合。我们发现,在压缩条件下,一级共价 I-O 键和二级卤素 I-O 相互作用逐渐向 O-I-O 缺电子多中心键转变。因此,碘超配位将 IO3 三角金字塔转化为 IO6 单元。这些 IO6 单元的形成打破了碘酸盐分子的典型隔离状态,形成了一个无限的二维碘酸盐网络。超配位对氢原子也有影响,因此多中心 O-H-O 键也会随着压力的增加而增强。我们已经确定,K2Zn(IO3)4-2H2O 是迄今为止研究过的最具可压缩性的碘酸盐之一,其体积模量为 22(3) GPa。光吸收测量和带状结构计算表明,压力引起的结构变化强烈改变了电子结构。带隙能从环境压力下的 4.2(1) eV 下降到 20 GPa 时的 3.4(1) eV。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


