Effect of Electrical Double Layer on Stability Mechanism of the Cluster of Bulk Nanobubbles
IF 3.9
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nanobubbles (NBs) hold significant promise in the fields of water treatment and environmental remediation due to their remarkable stability and longevity. Despite evidence of the stability of bulk nanobubbles (BNBs) in water, the underlying mechanisms of their stability remain elusive, with a notable gap in understanding the role of surface electronegativity in NBs’ stability. In this work, an all-atom (AA) molecular dynamics (MD) simulation has been used to investigate the stability characteristics of individual BNBs and the aggregation behavior of double BNB clusters, incorporating the influence of the electrical double layer (EDL). The stability of individual BNBs is evaluated through analysis of the gas–liquid interface’s high-density layer, the structure of the EDL, and the hydrogen bond (HB) network. A stabilization mechanism is proposed based on the surface electronegativity of BNBs. Meanwhile, the simultaneous construction of a double BNBs stability model reveals that nanobubble aggregation is the result of a competitive mechanism of van der Waals gravity and electrostatic repulsion. The validity of the proposed model is also verified by comparing the particle size and zeta tests of BNB solutions prepared with two gases with the nanobubble diameters and electrostatic energy obtained from the simulation model. A critical distance of 10 Å is determined, beyond which BNBs are less likely to coalesce. It is observed that the majority of BNBs are influenced by significantly greater electrostatic forces compared to the van der Waals force, which is hypothesized to be the main contributor to their stability.
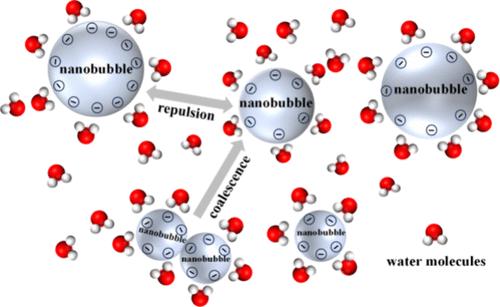
双电层对体纳米气泡团簇稳定机理的影响
纳米气泡由于其优异的稳定性和寿命,在水处理和环境修复领域具有重要的应用前景。尽管有证据表明体积纳米气泡(BNBs)在水中具有稳定性,但其稳定性的潜在机制仍然难以捉摸,在理解表面电负性在纳米气泡稳定性中的作用方面存在显着差距。本文采用全原子(AA)分子动力学(MD)模拟方法,研究了考虑双电层(EDL)影响的单个BNB的稳定性特征和双BNB簇的聚集行为。通过分析气液界面高密度层、EDL结构和氢键(HB)网络来评价单个bnb的稳定性。提出了一种基于BNBs表面电负性的稳定机理。同时,双BNBs稳定性模型的建立揭示了纳米气泡聚集是范德华引力和静电斥力竞争机制的结果。通过对比两种气体制备的BNB溶液的粒径和zeta测试,以及仿真模型得到的纳米气泡直径和静电能,验证了所提模型的有效性。确定了10 Å的临界距离,超过这个距离bnb就不太可能合并。可以观察到,与范德华力相比,大多数bnb受到更大的静电力的影响,假设这是其稳定性的主要贡献者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


