Role of Surface Oxygen in α-MoC Catalyst Stability and Activity under Electrooxidation Conditions
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
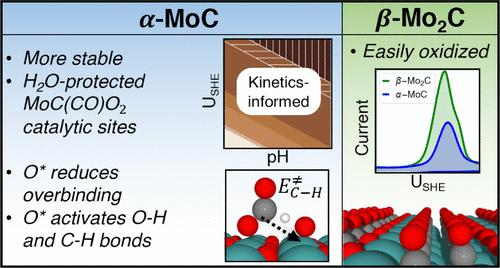
Transition metal carbides are attractive, low-cost alternatives to Pt group metals, exhibiting multifunctional acidic, basic, and metallic sites for catalysis. Their widespread applications are often impeded by a high surface affinity for oxygen, which blocks catalytic sites. However, recent reports indicate that the α-MoC phase is a stable and effective cocatalyst for reactions in oxidative or aqueous environments. In this work, we elucidate the factors affecting the stability and catalytic activity of α-MoC under mild electrooxidation conditions (0–0.8 V SHE) using density functional theory calculations, kinetics-informed surface Pourbaix diagram analysis, electronic structure analysis, and cyclic voltammetry. Both computational and experimental data indicate that α-MoC is significantly more resistant to electrooxidation by H2O than β-Mo2C. This higher stability is attributed to structural and kinetic factors, as the Mo-terminated α-MoC surface disfavors substitutional oxidation of partially exposed, less oxophilic C* atoms by hindering CO/CO2 removal. The α-MoC surface exposes H2O-protected [MoC2O2] and [MoC(CO)O2] oxycarbidic motifs available for catalysis in a wide potential window. At higher potentials, they convert to unstable [Mo(CO)2O2], resulting in material degradation. Using formic acid as a probe molecule, we obtain evidence for Pt-like O*-mediated O–H and C–H bond activation pathways. The largest kinetic barrier, observed for the C–H bond activation, correlates with the hydrogen affinity of the site in the order O*/Mofcc > O*/Ctop > O*/Motop. To mitigate the site-blocking effect of surface-bound H2O and bidentate formate, doping with Pt was investigated computationally to make the surface less oxophilic and more carbophilic, indicating a possible design strategy toward more active and selective carbide electrocatalysts.
表面氧对α-MoC催化剂电氧化稳定性和活性的影响
过渡金属碳化物是铂族金属的有吸引力的低成本替代品,具有多功能的酸性、碱性和金属催化作用。它们的广泛应用往往受到表面对氧的高亲和力的阻碍,这阻碍了催化位点。然而,最近的报道表明,α-MoC相是氧化或水环境中反应的稳定有效的助催化剂。本文采用密度泛函理论计算、表面Pourbaix图分析、电子结构分析和循环伏安法研究了α-MoC在温和电氧化条件(0-0.8 V SHE)下稳定性和催化活性的影响因素。计算和实验数据均表明,α-MoC比β-Mo2C更能抵抗H2O的电氧化。这种较高的稳定性归因于结构和动力学因素,因为mo端α-MoC表面阻碍了CO/CO2的去除,从而阻碍了部分暴露的、不太亲氧的C*原子的取代氧化。α-MoC表面暴露出受h2o保护的[MoC2O2]和[MoC(CO)O2]氧化基序,可在宽电位窗口内催化。在高电位下,它们转化为不稳定的[Mo(CO)2O2],导致材料降解。使用甲酸作为探针分子,我们获得了pt样O*介导的O - h和C-H键激活途径的证据。在C-H键活化过程中,观察到的最大的动力学势垒与位点的氢亲和性有关,其顺序为O*/Mofcc >;O * / Ctop祝辞O * / Motop。为了减轻表面结合的H2O和双齿甲酸酯的位点阻断效应,研究人员通过计算研究了铂的掺杂,使表面亲氧性降低,亲碳性提高,这表明了一种可能的设计策略,即更有活性和选择性的碳化物电催化剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


