Recognition of GC base pairs of B-DNA by coumarin-based benzimidazopyrimidines†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A novel series of Fe(iii)-catalyzed crescent-shaped coumarin-appended benzo[4,5]imidazo[1,2-a]pyrimidines has been generated using a single-step multi-component approach that includes a coumarin-derived β keto ester, 2-aminobenzimidazole, and various aldehydes. The mild eco-friendly reaction conditions allowed us, for the first time, to construct a library of highly substituted benzo[4,5]imidazo[1,2-a]pyrimidine (CBPy) heterocycles with a wide range of substrate compatibility and excellent yields. This one-pot synthesis is green in nature and conforms to atom economy. The structure of one representative compound () was established by X-ray crystallographic analysis. Our designed CBPys are bent in shape and capable of fitting into the minor groove of the B-DNA structure. Among all the CBPys, compound exhibited the strongest binding interaction (Kd = 2.9 μM) with calf thymus DNA (ctDNA), which is known to form the B-DNA structure under the experimental conditions. A competitive binding study confirmed that the location of was 43.77 Å away from the AT-rich region in the minor groove of B-DNA. It was also established that the crescent shape and the presence of coumarin were crucial for the binding of CBPys with B-DNA structures. Our results with DNA oligonucleotides of variable GC content suggest that compound specifically recognizes and binds to the GC base pairs of the B-DNA structure. Thus, the CBPy class of molecules may open a new avenue for the development of novel therapeutic drugs through GC recognition.
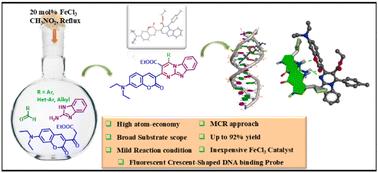
香豆素基苯并咪唑嘧啶对B-DNA GC碱基对的识别。
采用单步多组分方法合成了一系列新的Fe(III)催化的新月形香豆素附加苯并[4,5]咪唑[1,2- A]嘧啶,包括香豆素衍生的β酮酯,2-氨基苯并咪唑和各种醛。在温和的生态友好的反应条件下,我们首次构建了具有广泛底物相容性和优异产率的高取代苯并[4,5]咪唑[1,2-a]嘧啶(CBPy)杂环文库。这种一锅法合成自然绿色,符合原子经济。通过x射线晶体学分析确定了其中一个代表性化合物(4a)的结构。我们设计的CBPys具有弯曲的形状,能够适应B-DNA结构的小凹槽。在所有CBPys中,化合物4a与小牛胸腺DNA (ctDNA)的结合作用最强(Kd = 2.9 μM),在实验条件下已知形成B-DNA结构。通过竞争结合研究证实,4a的位置在B-DNA小槽中,距离at富集区43.77 Å。研究还发现,新月形状和香豆素的存在对于CBPys与B-DNA结构的结合至关重要。我们对可变GC含量的DNA寡核苷酸的研究结果表明,化合物4a特异性地识别并结合B-DNA结构的GC碱基对。因此,CBPy类分子可以通过GC识别为开发新型治疗药物开辟新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


