Novel low Cr-containing alloys for intermediate temperature solid oxide electrochemical devices
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The most popular alloys for solid oxide fuel cells (SOFC) and solid oxide electrolysis cells (SOEC) interconnects are ferritic stainless steels such as Crofer 22 APU with high chromium contents of more than 22 wt%. The main problem with these steels is that in oxidising atmospheres under SOFC operating conditions, chromium evaporates from them, causing electrode degradation. The possibility of solving this problem by creating precision alloys with low chromium content and a certain level of electrophysical and mechanothermal properties is discussed. This work presents novel alloys based on Ni-Mo and Co-Fe systems with a chromium content of no more than 6 wt%. It has been shown that the alloys meet the requirements of oxidation resistance and mechanothermal compatibility with electrolytes based on cerium dioxide and lanthanum gallate for intermediate temperature solid oxide electrochemical devices. Properties of these alloys such as linear thermal expansion, oxidation resistance and specific resistance meet the requirements for materials of intermediate temperature solid oxide electrochemical devices in the operating temperature range. This fact allows us to consider the alloys as promising materials for use as interconnects of SOFCs and SOECs, but further research is required.
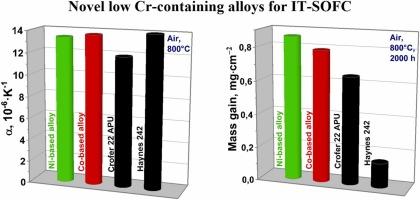
用于中温固体氧化物电化学装置的新型低含铬合金
用于固体氧化物燃料电池(SOFC)和固体氧化物电解电池(SOEC)互联器的最常用合金是铁素体不锈钢,例如铬含量超过 22 重量%的 Crofer 22 APU。这些钢材的主要问题是,在 SOFC 运行条件下的氧化气氛中,铬会从钢材中蒸发,导致电极降解。本文讨论了通过制造铬含量低且具有一定电物理和机械热性能的精密合金来解决这一问题的可能性。本研究提出了基于镍-钼和钴-铁体系的新型合金,其铬含量不超过 6 wt.%。研究表明,这些合金符合中温固体氧化物电化学装置对抗氧化性和与基于二氧化铈和没食子酸镧的电解质的机械热兼容性的要求。这些合金的线性热膨胀、抗氧化性和比电阻等特性符合中温固体氧化物电化学装置在工作温度范围内对材料的要求。因此,我们认为这些合金有望用作 SOFC 和 SOEC 的互连材料,但仍需进一步研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


