HER2 testing: evolution and update for a companion diagnostic assay
IF 82.2
1区 医学
Q1 ONCOLOGY
引用次数: 0
Abstract
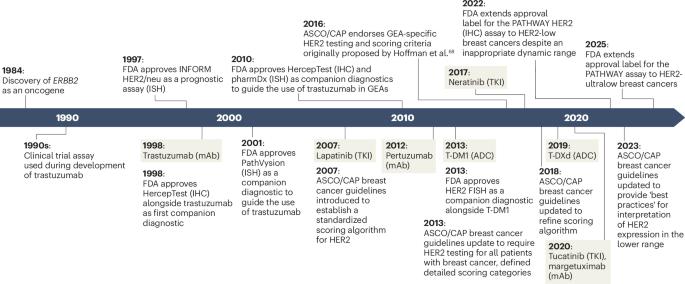
Human epidermal growth factor receptor 2 (HER2; encoded by ERBB2) testing has been a cornerstone of patient selection for HER2-targeted therapies, principally in breast cancer but also in several other solid tumours. Since the introduction of HercepTest as the original companion diagnostic for trastuzumab, HER2 assessment methods have evolved substantially, incorporating various testing modalities, from western blots, immunohistochemistry and fluorescence in situ hybridization, to early chromogenic quantitative methods and, probably in the future, fully quantitative methods. The advent of highly effective HER2-targeted antibody–drug conjugates with clinical activity at low levels of HER2 expression, such as trastuzumab deruxtecan, has necessitated the re-evaluation of HER2 testing, particularly for HER2-low tumours. In this Review, we provide an in-depth overview of the evolution of HER2 testing, the current clinical guidelines for HER2 testing across various solid tumours, challenges associated with current testing methodologies and the emerging potential of quantitative techniques. We discuss the importance of accurately defining HER2-low expression for therapeutic decision-making and how newer diagnostic approaches, such as quantitative immunofluorescence and RNA-based assays, might address the limitations of traditional immunohistochemistry-based methods. As the use of HER2-targeted therapies continues to expand to a wider range of tumour types, ensuring the precision and accuracy of HER2 testing will be crucial for guiding treatment strategies and improving patient outcomes. The development of companion diagnostic assays enabling the detection and quantification of HER2 expression and ERBB2 amplifications has provided an important early example that has informed the development of many companion diagnostics for the identification of other drug targets. However, the introduction of trastuzumab deruxtecan and, potentially, other novel antibody–drug conjugates has created the need for assays that are much more sensitive. In this Review, the authors describe the historical development of HER2 companion diagnostic assays in breast and other HER2-positive cancers and highlight the possible, future, more sensitive methods of HER2 detection and quantification that might provide the next generation of companion diagnostics.

HER2检测:伴随诊断试验的发展和更新
人表皮生长因子受体2;ERBB2编码)检测一直是患者选择her2靶向治疗的基础,主要用于乳腺癌,但也用于其他几种实体肿瘤。自从HercepTest作为曲妥珠单抗最初的伴随诊断引入以来,HER2评估方法已经发生了实质性的发展,包括各种检测方式,从免疫印迹、免疫组织化学和荧光原位杂交,到早期显色定量方法,以及可能在未来的完全定量方法。高效的HER2靶向抗体-药物偶联物的出现,在低水平HER2表达下具有临床活性,如曲妥珠单抗德鲁西替康,使得重新评估HER2检测成为必要,特别是对于HER2低水平的肿瘤。在这篇综述中,我们深入概述了HER2检测的发展,各种实体肿瘤中HER2检测的当前临床指南,与当前检测方法相关的挑战以及定量技术的新兴潜力。我们讨论了准确定义her2低表达对治疗决策的重要性,以及新的诊断方法,如定量免疫荧光和基于rna的检测,如何解决传统基于免疫组织化学方法的局限性。随着HER2靶向治疗的使用不断扩展到更广泛的肿瘤类型,确保HER2检测的精确性和准确性对于指导治疗策略和改善患者预后至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


