A novel acenaphthoimidazolyidene oxazolinic palladium complex and its efficient catalysis in Suzuki–Miyaura cross-coupling reactions of N-acyl-glutarimides via N–C cleavage†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, we synthesized a new Pd–N-heterocyclic carbene (Pd–NHC) complex that featured an acenaphthoimidazolylidene (AnIm) skeleton and 2-phenyl-2-oxazoline. This catalyst exhibited extremely high efficiency in Suzuki–Miyaura couplings between N-acyl-glutarimides and organoboronic acids, affording various aryl ketones in excellent yields with a broad substrate scope and wide functional group compatibility. Notably, the reactions were completed in just 5 hours with only 0.5 mol% of catalyst. In addition, this catalytic system also enables the efficient synthesis of functional molecular intermediates. The catalyst, designed through the synergistic integration of a throw-away ligand and an extended conjugated NHC system, further demonstrates its remarkable potential.
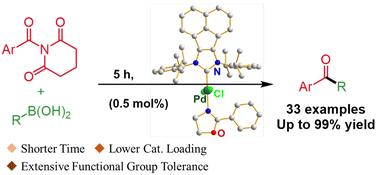
一种新型苊噻唑-恶唑啉钯配合物及其在n -酰基-戊二酰亚胺的N-C裂解铃木-宫浦交联反应中的催化作用。
在此,我们合成了一个新的pd - n -杂环羰基(Pd-NHC)配合物,该配合物具有苊噻唑咪唑(AnIm)骨架和2-苯基-2-恶唑啉。该催化剂在n -酰基戊二酰亚胺与有机硼酸之间的Suzuki-Miyaura偶联反应中表现出极高的效率,以优异的产率生成各种芳基酮,具有广泛的底物范围和广泛的官能团相容性。值得注意的是,在催化剂用量仅为0.5 mol%的情况下,反应在5小时内完成。此外,该催化体系还能高效合成功能分子中间体。该催化剂通过一次性配体和扩展共轭NHC体系的协同整合而设计,进一步展示了其巨大的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


