Electrochemically driven reductive cyclization of o-nitroanilines: synthesis of 1,2-fused benzimidazoles and benzo[d]imidazoles†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The electrochemical synthesis of 1,2-fused benzimidazoles and benzo[d]imidazoles from o-nitroanilines in an undivided cell under constant current conditions was developed. The electrosynthesis proceeded through a tandem process involving nitro reduction/C(sp3)–H amination/condensation. The method can accommodate a broad range of o-nitroanilines and results in the desired products in yields of up to 99%. A plausible reaction mechanism was proposed on the basis of controlled experiments and cyclic voltammetry (CV) analysis. The benefits of the developed method include one-pot synthesis, open-air conditions, gram-scale synthesis and no requirement for a strong reductant.
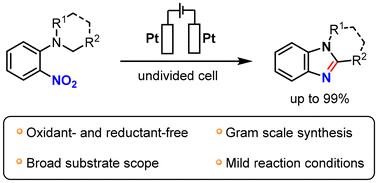
邻硝基苯胺的电化学还原环化:1,2-熔融苯并咪唑和苯并[d]咪唑的合成。
在恒定电流条件下,开发了在不分流电池中以邻硝基苯胺为原料电化学合成 1,2-融合苯并咪唑和苯并[d]咪唑的方法。电合成是通过硝基还原/C(sp3)-H 氨基化/缩合的串联过程进行的。该方法适用于多种邻硝基苯胺,并能得到所需的产物,产率高达 99%。在对照实验和循环伏安法(CV)分析的基础上,提出了一个合理的反应机制。所开发方法的优点包括一锅合成、露天条件、克级合成以及无需强还原剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


