Chlamydia pneumoniae relies on host glutathione for its growth and induces integrated stress response-mediated changes in macrophage glutathione metabolism
IF 2.7
4区 医学
Q3 IMMUNOLOGY
引用次数: 0
Abstract
The obligate intracellular bacterium Chlamydia pneumoniae can enter into persistent phenotype, which is refractory to antibiotics and causes prolonged inflammatory state in the host. Molecular mechanisms enabling C. pneumoniae intracellular survival and governing the balance between persistent and productive infection phenotype remain poorly understood. In this study, the role of glutathione (GSH) metabolism in C. pneumoniae growth and progeny production was studied in THP-1 macrophages and A549 epithelial cells. Results indicate that depletion of cellular GSH pools decreased C. pneumoniae replication, but only if the constituent amino acids were also sequestered from the culture. C. pneumoniae infection increased the expression of GSH biosynthetic genes but also upregulated ChaC1, an intracellular enzyme involved in GSH breakage. C. pneumoniae infection was found to increase PERK phosphorylation in THP-1 macrophages and chemical inhibition of PERK prevented the infection-induced upregulation of GSH biosynthesis and GSH degradation genes and suppressed C. pneumoniae replication. C. pneumoniae -induced ChaC1 upregulation was also suppressed by protein kinase R inhibitor or treatment with ISRIB, indicating an involvement of redundant pathways of the host cell stress response. The data suggest that C. pneumoniae requires amino acids derived from the host cell GSH pools to enable active bacterial replication.
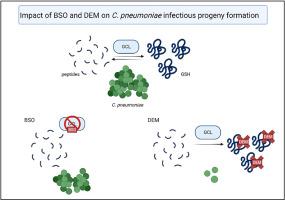
肺炎衣原体依赖宿主谷胱甘肽生长并诱导巨噬细胞谷胱甘肽代谢的综合应激反应介导的变化。
专性细胞内细菌肺炎衣原体可进入持久性表型,对抗生素难以耐受,导致宿主的炎症状态延长。使肺炎原胞内存活和控制持续性和生产性感染表型之间的平衡的分子机制仍然知之甚少。本研究在THP-1巨噬细胞和A549上皮细胞中研究谷胱甘肽(GSH)代谢在肺炎球菌生长和后代产生中的作用。结果表明,细胞GSH池的耗竭减少了肺炎球菌的复制,但仅当组成氨基酸也从培养物中分离出来时。肺炎球菌感染增加了GSH生物合成基因的表达,但也上调了ChaC1的表达,ChaC1是一种参与GSH破坏的细胞内酶。发现肺炎C.感染可增加THP-1巨噬细胞PERK磷酸化,化学抑制PERK可阻止感染诱导的GSH生物合成和GSH降解基因上调,抑制肺炎C.复制。蛋白激酶R抑制剂或ISRIB治疗也能抑制肺炎原体诱导的ChaC1上调,表明参与了宿主细胞应激反应的冗余途径。这些数据表明,肺炎球菌需要来自宿主细胞GSH池的氨基酸来激活细菌复制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microbes and Infection
医学-病毒学
CiteScore
12.60
自引率
1.70%
发文量
90
审稿时长
40 days
期刊介绍:
Microbes and Infection publishes 10 peer-reviewed issues per year in all fields of infection and immunity, covering the different levels of host-microbe interactions, and in particular:
the molecular biology and cell biology of the crosstalk between hosts (human and model organisms) and microbes (viruses, bacteria, parasites and fungi), including molecular virulence and evasion mechanisms.
the immune response to infection, including pathogenesis and host susceptibility.
emerging human infectious diseases.
systems immunology.
molecular epidemiology/genetics of host pathogen interactions.
microbiota and host "interactions".
vaccine development, including novel strategies and adjuvants.
Clinical studies, accounts of clinical trials and biomarker studies in infectious diseases are within the scope of the journal.
Microbes and Infection publishes articles on human pathogens or pathogens of model systems. However, articles on other microbes can be published if they contribute to our understanding of basic mechanisms of host-pathogen interactions. Purely descriptive and preliminary studies are discouraged.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


