Chemical activation of willow with co-presence of FeCl3 tailors pore structure of activated carbon for enhanced adsorption of phenol and tetracycline
IF 8
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
FeCl3 is a Lewis acid catalyzing condensation reaction, which might be beneficial for enhancing mass yield of activated carbon (AC) if used as a co-activator. Herein, this was verified by conducting activation of willow with various activators (ZnCl2, K2C2O4, H3PO4) in the presence/absence of FeCl3. The results indicated that FeCl3 competed with acid-catalyzed reactions induced by ZnCl2 or H3PO4, interfering aromatization and diminishing AC yields (from 40.8 % to 37.0 % with ZnCl2). In the activation with K2C2O4 + FeCl3, In-situ IR measurement showed that FeCl3 catalyzed polymerization reactions, but polymeric products were not stable and were further cracked with K2C2O4, reducing AC yield drastically from 27.6 % to 7.1 %. The co-presence of FeCl3 in the activation reduced overall specific surface area (1201.6 versus 1127.2 m2 g−1 for K2C2O4) by merging of micropores to form a higher percentage of mesopores (i.e. 2.0 % to 17.1 % for K2C2O4, and 9.2 % to 41.4 % for H3PO4). This pore restructuring significantly enhanced tetracycline adsorption (99.9 % removal for AC- K2C2O4 + FeCl3 versus 61.1 % for AC-K2C2O4 alone), while compromising phenol adsorption (48.7 % versus 96.1 %) due to reduced micropore availability. The reduced specific surface area was also attributed to the retention of inorganics by solid phase reactions between FeCl3 and K2C2O4 or H3PO4. Additionally, the presence of FeCl3 resulted in more fragmented surface of ACs generated from all activators.
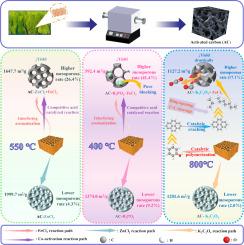
化学活化柳树与FeCl3共存在裁缝孔结构的活性炭对苯酚和四环素的吸附增强
FeCl3是Lewis酸催化缩合反应,作为助活化剂可能有利于提高活性炭的质量产率。本文通过在FeCl3存在/不存在的情况下,用各种活化剂(ZnCl2、K2C2O4、H3PO4)对柳树进行活化,验证了这一点。结果表明,FeCl3与ZnCl2或H3PO4诱导的酸催化反应竞争,干扰芳香化,使AC收率从40.8%降低到37.0%。在K2C2O4 + FeCl3的活化下,原位红外测量表明,FeCl3催化聚合反应,但聚合产物不稳定,并被K2C2O4进一步裂解,AC收率从27.6%大幅降低至7.1%。FeCl3在活化过程中的共同存在通过合并微孔形成更高比例的介孔(即K2C2O4的2.0%至17.1%,H3PO4的9.2%至41.4%),降低了总比表面积(K2C2O4的1201.6对1127.2 m2 g−1)。这种孔重组显著增强了四环素的吸附(AC-K2C2O4 + FeCl3的去除率为99.9%,而AC-K2C2O4单独去除率为61.1%),同时由于微孔可用性降低,苯酚的吸附(48.7%,而AC-K2C2O4单独去除率为96.1%)降低。由于FeCl3与K2C2O4或H3PO4的固相反应,无机物的滞留也导致了比表面积的减小。此外,FeCl3的存在导致所有活化剂产生的ACs表面碎片化程度更高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science of the Total Environment
环境科学-环境科学
CiteScore
17.60
自引率
10.20%
发文量
8726
审稿时长
2.4 months
期刊介绍:
The Science of the Total Environment is an international journal dedicated to scientific research on the environment and its interaction with humanity. It covers a wide range of disciplines and seeks to publish innovative, hypothesis-driven, and impactful research that explores the entire environment, including the atmosphere, lithosphere, hydrosphere, biosphere, and anthroposphere.
The journal's updated Aims & Scope emphasizes the importance of interdisciplinary environmental research with broad impact. Priority is given to studies that advance fundamental understanding and explore the interconnectedness of multiple environmental spheres. Field studies are preferred, while laboratory experiments must demonstrate significant methodological advancements or mechanistic insights with direct relevance to the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


