Lanthanide-Selective Artificial Channels
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
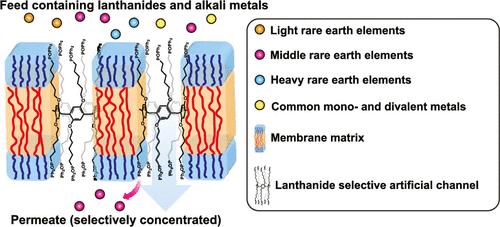
Lanthanides serve as essential elements for modern technology, playing critical roles in batteries, wind turbines, portable electronics, and energy-efficient lighting. Purifying lanthanides from ores and recycling them from end-of-life consumer materials are costly and damaging to the environment due to inefficient separation technologies. In this study, we present a new approach for lanthanide separations using supramolecular membrane channel nanopores based on a pillar[5]arene scaffold with appended diphenylphosphine oxide (DPP) ligands. These channels show high transport selectivity (>18:1) of the middle lanthanides, europium (Eu3+) and terbium (Tb3+) ions, over monovalent K+ ions and also excluded other common mono- and divalent metal ions (Na+, Ca2+, and Mg2+) including protons. These membrane channels also have high lanthanide–lanthanide transport selectivity with Eu3+/La3+ selectivity of >40 and Eu3+/Yb3+ selectivity of ∼30. Additionally, they demonstrated significantly higher selectivities between middle lanthanides and both light and heavy lanthanides: Tb3+/La3+ (∼140), Tb3+/Yb3+ (∼72), Tb3+/Nd3+ (∼58), and Eu3+/Nd3+ (∼17), which are considerably higher than selectivities reported in studies using traditional solvent extraction methods. Molecular dynamics simulations indicate that the high selectivity observed is due to specific water-mediated interactions between the hydrated ions and the channel. Our findings could contribute to ongoing efforts to improve lanthanide separation efficiency and reduce the environmental impact associated with current methods.
镧系元素选择性人工通道
镧系元素是现代技术的基本元素,在电池、风力涡轮机、便携式电子设备和节能照明中发挥着关键作用。从矿石中提纯镧系元素并从报废的消费材料中回收镧系元素成本高昂,而且由于分离技术效率低下,对环境造成了破坏。在这项研究中,我们提出了一种基于附加二苯基氧化膦(DPP)配体的柱状[5]芳烃支架的超分子膜通道纳米孔分离镧系元素的新方法。这些通道对中间镧系元素,铕(Eu3+)和铽(Tb3+)离子的传输选择性(>18:1)高于单价K+离子,也排除了其他常见的一价和二价金属离子(Na+, Ca2+和Mg2+),包括质子。这些膜通道还具有较高的镧系元素-镧系元素传输选择性,Eu3+/La3+选择性为40,Eu3+/Yb3+选择性为30。此外,它们在中镧系元素与轻镧系元素和重镧系元素之间表现出明显更高的选择性:Tb3+/La3+ (~ 140), Tb3+/Yb3+ (~ 72), Tb3+/Nd3+(~ 58)和Eu3+/Nd3+(~ 17),这大大高于使用传统溶剂萃取方法研究报告的选择性。分子动力学模拟表明,观察到的高选择性是由于水合离子和通道之间特定的水介导的相互作用。我们的发现有助于提高镧系元素的分离效率,减少现有方法对环境的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


