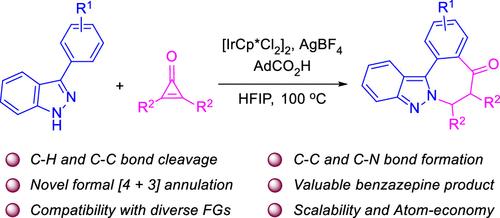
Synthesis of Indazole Fused 2-Benzazepines with Polarity-Dependent Fluorescence Based on Formal [4 + 3] Annulation of 3-Aryl-1H-indazoles with Cyclopropenones
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The effective assembly of benzazepine skeletons in a sustainable and atom-economical fashion remains a challenging goal in modern organic synthesis. Presented herein is a novel synthesis of indazole fused 2-benzazepine derivatives based on a formal [4 + 3] annulation of 3-aryl-1H-indazoles with cyclopropenones. The formation of products proceeds through Ir(III)-catalyzed aryl C–H bond metalation and cyclopropenone ring-opening leading to aryl acylation, followed by an intramolecular N-nucleophilic conjugated addition. By using this method, a number of valuable benzazepine derivatives were effectively generated. This protocol addresses the challenges in constructing medium-sized rings through cascade C–H/C–C bond activation and C–C/C–N bond formation. Moreover, the photophysical properties of the products thus obtained were also evaluated. It turned out that all compounds tested showed solvent polarity-dependent fluorescence features, which could be potentially applied for revealing the polarity of their immediate environments.
基于3-芳基- 1h -吲哚与环丙烯[4 + 3]环的极性依赖荧光茚唑融合2-苯并杂氮类化合物的合成
在现代有机合成中,以可持续和原子经济的方式有效地组装苯氮平骨架仍然是一个具有挑战性的目标。本文以3-芳基- 1h -吲哚与环丙烯形成[4 + 3]环为基础,提出了一种新的吲哚- 2-苯并氮衍生物的合成方法。产物的形成是通过Ir(III)催化芳基C-H键金属化和环丙烯开环导致芳基酰化,然后是分子内亲n核共轭加成。通过这种方法,有效地合成了许多有价值的苯扎平类衍生物。该方案解决了通过级联C-H / C-C键激活和C-C / C-N键形成构建中型环的挑战。并对所得产物的光物理性质进行了评价。结果表明,所有测试的化合物都显示出溶剂极性依赖的荧光特征,这可能被用于揭示其直接环境的极性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


