Bioenzyme Inspired Heterointerface Construction of NiFeSe/Ni3S2 for Improved Overall Water Splitting
IF 4.7
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Electrocatalytic water splitting for hydrogen production represents a crucial pathway toward establishing sustainable energy infrastructure and addressing environmental concerns, with the development of high-performance nonprecious metal catalysts being a central focus. While Ni3S2 demonstrates potential as an electrocatalyst, its limited functionality and suboptimal performance necessitate further enhancement. In this study, drawing inspiration from natural hydrogenases, we engineered a novel NiFeSe/Ni3S2 composite electrocatalyst through the integration of NiFeSe with Ni3S2. The synthesized catalyst displayed outstanding overall water-splitting performance in alkaline media, realizing current densities of 100 and 10 mA cm–2 at remarkably low overpotentials of 267.4 mV (vs RHE) for oxygen evolution reaction (OER) and 105.6 mV (vs RHE) for hydrogen evolution reaction (HER), respectively. Remarkably, the two-electrode electrolyzer incorporating NiFeSe/Ni3S2 achieved a current density of 20 mA cm–2 at a substantially reduced cell voltage of 1.586 V. Comprehensive analysis revealed that the strategic construction of biomimetic active centers and heterogeneous interfaces significantly modulates the electronic structure, improved charge transfer, and redistribution of electron density of the catalytic sites. This investigation provides valuable insights and a promising framework for the rational design of high-performance bifunctional electrocatalysts for water-splitting applications.
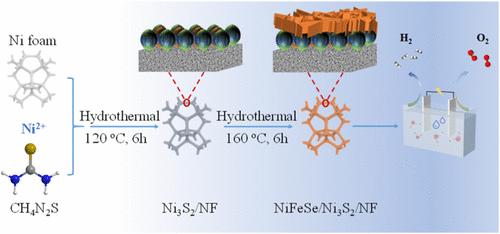
生物酶激发的NiFeSe/Ni3S2异质界面构建改善整体水分解
电催化水裂解制氢是建立可持续能源基础设施和解决环境问题的重要途径,高性能非贵金属催化剂的开发是一个核心焦点。虽然Ni3S2显示出作为电催化剂的潜力,但其有限的功能和不理想的性能需要进一步增强。在这项研究中,我们从天然氢化酶中获得灵感,通过将NiFeSe与Ni3S2整合,设计了一种新型的NiFeSe/Ni3S2复合电催化剂。合成的催化剂在碱性介质中表现出优异的整体解水性能,出氧反应(OER)和出氢反应(HER)的过电位分别为267.4 mV (vs RHE)和105.6 mV (vs RHE),电流密度分别为100和10 mA cm-2。值得注意的是,采用NiFeSe/Ni3S2的双电极电解槽在大幅降低的1.586 V电池电压下实现了20 mA cm-2的电流密度。综合分析表明,仿生活性中心和非均相界面的战略性构建显著调节了催化位点的电子结构,改善了电荷转移和电子密度的重新分布。这项研究为合理设计用于水分解的高性能双功能电催化剂提供了有价值的见解和有前途的框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


