I2-Induced Metal-Free C(sp2)–H Functionalization of Indoles via One-Pot and Two-Step Reaction with 1-(2-Hydroxyphenyl)-propargyl Alcohols: Access to 3-(Benzofuran-3-yl)-1H-indoles
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The I2-catalyzed reaction of 1-(2-hydroxyphenyl)-propargyl alcohols with indoles and subsequent treatment with K2CO3 provided (benzofuran-3-yl)-1H-indoles in good to excellent yields with high regioselectivity. This one-pot and two-step strategy proved to be suitable for a wide range of substrates except for aliphatic alkynyl alcohols as well as the indoles bearing N-protected groups such as the Ts group possessing strong electron-withdrawing feature. The reaction procedure involved a cross-coupling and the construction of a benzofuran framework via 5-exo-dig cyclization.
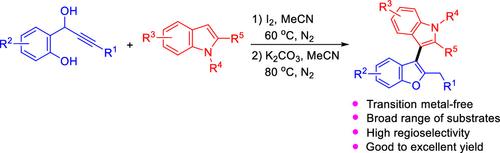
通过与1-(2-羟基苯基)-丙炔醇的一锅两步反应,i2诱导无金属C(sp2) - h功能化吲哚:获得3-(苯并呋喃-3-基)- 1h -吲哚
通过i2催化1-(2-羟基苯基)-丙炔醇与吲哚的反应以及随后的K2CO3处理,得到了收率高且具有高区域选择性的(苯并呋喃-3-基)- 1h -吲哚。这种一锅两步策略被证明适用于广泛的底物,除了脂肪族炔醇和含有n保护基团的吲哚,如具有强吸电子特征的Ts基团。反应过程包括交叉偶联和通过5-外显式环化构建苯并呋喃骨架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


