Sulfur-Controlled Modulation of Peptoid Atropisomeric Foldamers
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We incorporated the hetero atoms (O/S) at the ortho-position to investigate the steric influence on controlling the rotational barrier around the C–N chiral axis and to elucidate the chiral attributes of sulfur-containing N-aryl peptoids. This study reports the simultaneous installation of a C–N chiral axis and the integration of sulfur-containing stereogenic elements in peptoid atropisomeric foldamers. By leveraging multiple chiral elements in peptoids, we demonstrated subtle structural variations, particularly by varying the sulfur oxidation states, that can lead to significant differences in the rotational energy barrier, as determined by dynamic HPLC. Additionally, we employed single-crystal X-ray crystallography to elucidate local conformational ordering and computational studies to identify noncovalent interactions in this class of atropisomers. Through these combined approaches, we explored sulfur-controlled modulation of N-aryl peptoid atropisomeric foldamers.
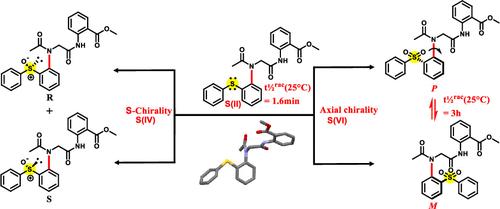
硫控制的肽类atrosomomer折叠体的调节
我们在邻位引入杂原子(O/S),以研究空间位对控制C-N手性轴周围旋转势垒的影响,并阐明含硫n -芳基类肽的手性性质。本研究报道了C-N手性轴的同时安装和含硫立体元素在类肽atroisomer折叠体中的整合。通过利用类肽中的多个手性元素,我们证明了微妙的结构变化,特别是通过改变硫氧化态,可以导致旋转能垒的显着差异,正如动态HPLC所确定的那样。此外,我们使用单晶x射线晶体学来阐明局部构象顺序和计算研究来确定这类atropisomers的非共价相互作用。通过这些结合的方法,我们探索了硫控制的n -芳基类肽atroisomer折叠体的调节。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


