Gut microbiome evolution from infancy to 8 years of age
IF 50
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
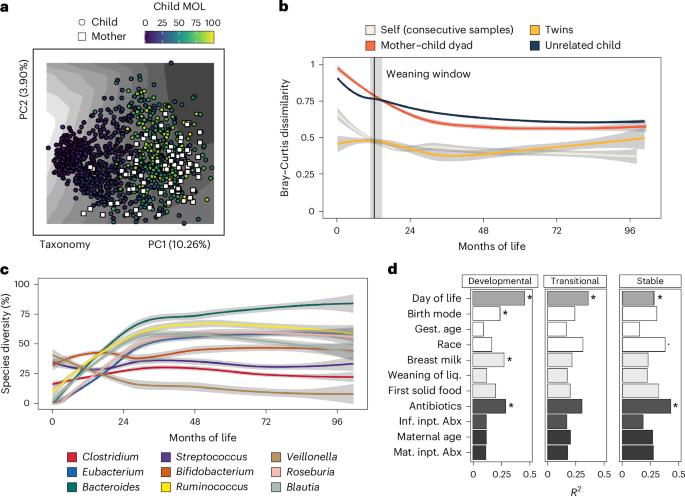
The human gut microbiome is most dynamic in early life. Although sweeping changes in taxonomic architecture are well described, it remains unknown how, and to what extent, individual strains colonize and persist and how selective pressures define their genomic architecture. In this study, we combined shotgun sequencing of 1,203 stool samples from 26 mothers and their twins (52 infants), sampled from childbirth to 8 years after birth, with culture-enhanced, deep short-read and long-read stool sequencing from a subset of 10 twins (20 infants) to define transmission, persistence and evolutionary trajectories of gut species from infancy to middle childhood. We constructed 3,995 strain-resolved metagenome-assembled genomes across 399 taxa, and we found that 27.4% persist within individuals. We identified 726 strains shared within families, with Bacteroidales, Oscillospiraceae and Lachnospiraceae, but not Bifidobacteriaceae, vertically transferred. Lastly, we identified weaning as a critical inflection point that accelerates bacterial mutation rates and separates functional profiles of genes accruing mutations. In a unique cohort of twins followed from birth to 8 years of age, shotgun sequencing of stool samples reveals that the transmission, persistence and evolutionary adaptation of bacterial strains are structured by early-life diet and weaning.
从婴儿到8岁的肠道微生物群进化
人类肠道微生物群在生命早期是最活跃的。尽管分类结构的全面变化已经被很好地描述,但个体菌株如何以及在多大程度上定植和持续存在以及选择压力如何定义它们的基因组结构仍然未知。在这项研究中,我们结合了来自26名母亲及其双胞胎(52名婴儿)的1,203份粪便样本的鸟枪测序,从分娩到出生后8年,以及来自10名双胞胎(20名婴儿)的培养增强,深度短读和长读粪便测序,以确定肠道物种从婴儿期到童年中期的传播,持久性和进化轨迹。我们在399个分类群中构建了3995个菌株分辨率的宏基因组组装基因组,我们发现27.4%的基因组在个体中持续存在。共鉴定出726株同科菌株,其中拟杆菌科、示波螺旋科和毛缕螺旋科垂直转移,双歧杆菌科不存在。最后,我们发现断奶是加速细菌突变率和分离突变基因功能谱的关键拐点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


