Breaking the linear scaling limit in multi-electron-transfer electrocatalysis through intermediate spillover
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
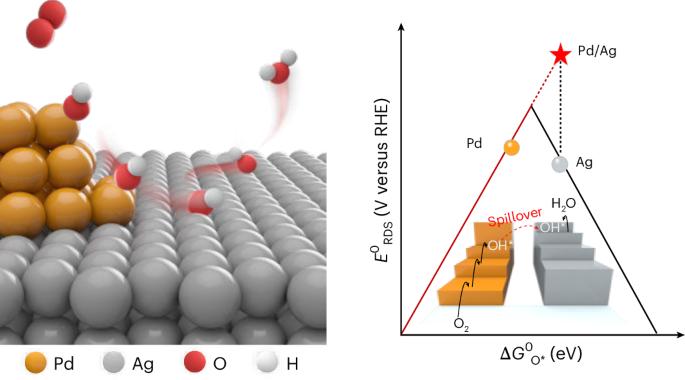
The linear scaling relationships between the adsorption energies of multiple intermediates constrain the maximum reaction activity of heterogeneous catalysis. Here we propose an intermediate spillover strategy to decouple the elementary electron-transfer steps in an electrochemical reaction by building a bi-component interface, thereby independently tuning the corresponding intermediate adsorption at an individual catalytic surface. Taking the electrocatalytic oxygen reduction reaction as an example, oxophilic sites are preferable for activating oxygen molecules, then the adsorbed OH* intermediates spontaneously migrate to the adjacent sites with a weaker oxygen binding energy, where OH* intermediates are further reduced and desorbed to complete the overall catalytic cycle. Consequently, the designed Pd/Ni(OH)2 catalyst can remarkably elevate the half-wave potential of the oxygen reduction reaction to ~70 mV higher than that of the Pt/C catalyst, surmounting the theoretical overpotential limit of Pd. This design principle highlights an opportunity for utilizing intermediate spillover to break the ubiquitous scaling relationships in multi-step catalytic reactions. Linear scaling relationships often hinder the optimization of heterogeneous catalysts. Now, an intermediate spillover strategy is put forward by designing bi-component catalysts, thereby decoupling elementary electron-transfer steps and circumventing linear scaling relationships.

通过中间溢出打破多电子转移电催化的线性结垢极限
多种中间体吸附能之间的线性标度关系制约了多相催化的最大反应活性。在这里,我们提出了一种中间溢出策略,通过建立双组分界面来解耦电化学反应中的基本电子转移步骤,从而在单个催化表面独立调节相应的中间吸附。以电催化氧还原反应为例,亲氧位点更有利于活化氧分子,被吸附的OH*中间体自发迁移到邻近氧结合能较弱的位点,OH*中间体进一步被还原解吸,完成整个催化循环。因此,Pd/Ni(OH)2催化剂能显著提高氧还原反应的半波电位,比Pt/C催化剂高~70 mV,突破了Pd的理论过电位极限。这种设计原则强调了利用中间溢出来打破多步催化反应中普遍存在的结垢关系的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


