Structural basis for membrane remodeling by the AP5–SPG11–SPG15 complex
IF 10.1
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
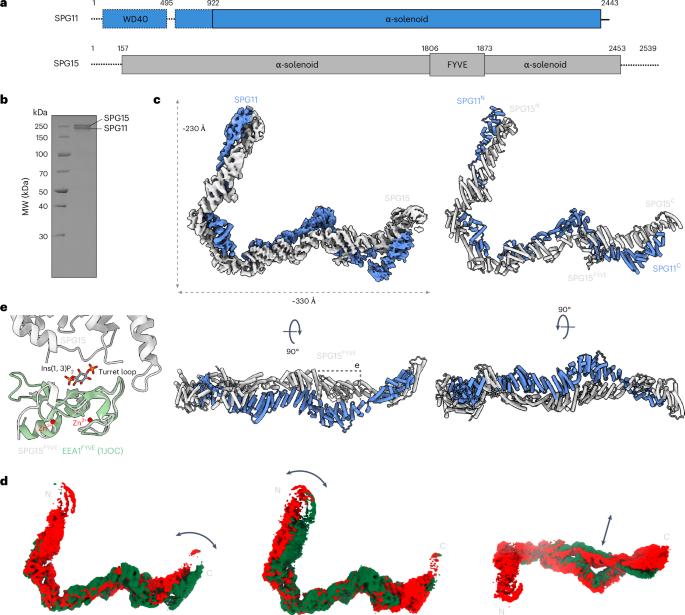
The human spastizin (spastic paraplegia 15, SPG15) and spatacsin (spastic paraplegia 11, SPG11) complex is involved in the formation of lysosomes, and mutations in these two proteins are linked with hereditary autosomal-recessive spastic paraplegia. SPG11–SPG15 can cooperate with the fifth adaptor protein complex (AP5) involved in membrane sorting of late endosomes. We employed cryogenic-electron microscopy and in silico predictions to investigate the structural assemblies of the SPG11–SPG15 and AP5–SPG11–SPG15 complexes. The W-shaped SPG11–SPG15 intertwined in a head-to-head fashion, and the N-terminal region of SPG11 is required for AP5 complex interaction and assembly. The AP5 complex is in a super-open conformation. Our findings reveal that the AP5–SPG11–SPG15 complex can bind PI3P molecules, sense membrane curvature and drive membrane remodeling in vitro. These studies provide insights into the structure and function of the spastic paraplegia AP5–SPG11–SPG15 complex, which is essential for the initiation of autolysosome tubulation. Mai and colleagues present cryo-electron microscopy structures of the SPG11–SPG15 and AP5–SPG11–SPG15 complex, offering insights into mechanisms of autophagic lysosome reformation and retrograde trafficking.

AP5-SPG11-SPG15复合物重塑膜的结构基础
人痉挛蛋白(spastizin, SPG15)和痉挛蛋白(spatacsin, SPG11)复合物参与溶酶体的形成,这两种蛋白的突变与遗传性常染色体隐性痉挛性截瘫有关。SPG11-SPG15可与第五接头蛋白复合物(AP5)协同参与后期核内体的膜分选。我们利用低温电子显微镜和硅预测研究了SPG11-SPG15和AP5-SPG11-SPG15配合物的结构组装。w形的SPG11 - spg15以头对头的方式缠绕在一起,SPG11的n端区域是AP5复合物相互作用和组装所必需的。AP5复合体呈超开放构象。我们的研究结果表明,AP5-SPG11-SPG15复合物可以结合PI3P分子,感知膜曲率并驱动膜重塑。这些研究提供了对痉挛截瘫AP5-SPG11-SPG15复合物的结构和功能的见解,该复合物对自溶酶体微管的起始至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


