From fat to filter: the effect of adipose tissue-derived signals on kidney function
IF 39.8
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
Abstract
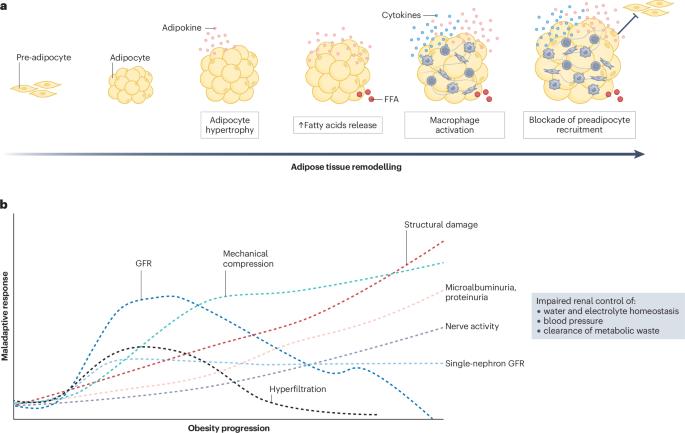
Obesity is associated with severe consequences for the renal system, including chronic kidney disease, kidney failure and increased mortality. Obesity has both direct and indirect effects on kidney health through several mechanisms, including activation of the renin–angiotensin system, mechanical compression, inflammation, fibrosis, increased filtration barrier permeability and renal nerve activity. The expansion of adipose tissue through hypertrophy and hyperplasia can induce haemodynamic changes that promote glomerular hyperfiltration to compensate for the greater metabolic demands of the increased body weight. Adipose expansion is also associated with the release of adipokines and pro-inflammatory cytokines, hyperinsulinaemia and insulin resistance, which exert direct and indirect effects on kidney function via various mechanisms. Increased uptake of fatty acids by the kidney leads to alterations in lipid metabolism and lipotoxicity, also contributing to the pro-inflammatory and pro-fibrotic environment. The role of the adipose tissue–brain–kidney axis in the obesity-associated decline in renal function is sustained by studies showing that stimulation of adipose tissue sensory neurons by locally released factors increases renal sympathetic nerve activity. Conversely, pre-existent kidney disease can contribute to adipose dysfunction through the accumulation of uraemic toxins and hormonal changes. These findings highlight the importance of crosstalk between adipose tissue and the kidneys and provide insights into the mechanisms underlying the associations between obesity and kidney disease. Obesity exerts both direct and indirect effects on the kidney. This Review describes the mechanisms by which adipose-derived signals, including neural and hormonal signals, compromise kidney filtration capacity and discusses current and emerging renoprotective therapies that may provide benefit in the context of obesity and metabolic disease.

从脂肪到过滤器:脂肪组织来源的信号对肾功能的影响
肥胖与肾脏系统的严重后果有关,包括慢性肾病、肾衰竭和死亡率增加。肥胖通过几种机制对肾脏健康有直接和间接的影响,包括肾素-血管紧张素系统的激活、机械压迫、炎症、纤维化、滤过屏障渗透性增加和肾神经活动。脂肪组织通过肥大和增生而扩张,可诱导血流动力学改变,促进肾小球高滤过,以补偿体重增加带来的更大代谢需求。脂肪扩张还与脂肪因子和促炎细胞因子的释放、高胰岛素血症和胰岛素抵抗有关,通过多种机制对肾功能产生直接和间接的影响。肾脏对脂肪酸的摄取增加导致脂质代谢和脂毒性的改变,也有助于促炎和促纤维化的环境。脂肪组织-脑-肾轴在肥胖相关的肾功能下降中的作用得到了研究的支持,研究表明,局部释放的因子刺激脂肪组织感觉神经元会增加肾交感神经的活动。相反,先前存在的肾脏疾病可以通过尿毒毒素的积累和激素的变化来促进脂肪功能障碍。这些发现强调了脂肪组织和肾脏之间相互作用的重要性,并为肥胖和肾脏疾病之间关联的潜在机制提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews Nephrology
医学-泌尿学与肾脏学
CiteScore
39.00
自引率
1.20%
发文量
127
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Nephrology aims to be the premier source of reviews and commentaries for the scientific communities it serves.
It strives to publish authoritative, accessible articles.
Articles are enhanced with clearly understandable figures, tables, and other display items.
Nature Reviews Nephrology publishes Research Highlights, News & Views, Comments, Reviews, Perspectives, and Consensus Statements.
The content is relevant to nephrologists and basic science researchers.
The broad scope of the journal ensures that the work reaches the widest possible audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


