Synthesis, structure, and properties of antiaromatic PVO complexes of triphyrins(2.1.1)
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
A series of antiaromatic stable PV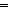 O complexes of meso-tetra(p-tolyl) triphyrin(2.1.1) and its β-substituted derivatives were synthesized in 38–48% yields by treating an appropriate aromatic free base triphyrin(2.1.1) with PCl3 in toluene/Et3N at reflux for 4–6 h. During the reaction, 14π aromatic meso-tetra(p-tolyl) triphyrin(2.1.1) is reduced to a 16π macrocycle and complexes with PV
O complexes of meso-tetra(p-tolyl) triphyrin(2.1.1) and its β-substituted derivatives were synthesized in 38–48% yields by treating an appropriate aromatic free base triphyrin(2.1.1) with PCl3 in toluene/Et3N at reflux for 4–6 h. During the reaction, 14π aromatic meso-tetra(p-tolyl) triphyrin(2.1.1) is reduced to a 16π macrocycle and complexes with PV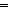 O to form antiaromatic PV
O to form antiaromatic PV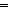 O complexes of meso-tetra(p-tolyl) triphyrins(2.1.1). The X-ray structure obtained for one of the PV
O complexes of meso-tetra(p-tolyl) triphyrins(2.1.1). The X-ray structure obtained for one of the PV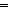 O triphyrin(2.1.1) complexes revealed that unlike the almost planar structure of free base meso-tetraaryl triphyrin(2.1.1), PV
O triphyrin(2.1.1) complexes revealed that unlike the almost planar structure of free base meso-tetraaryl triphyrin(2.1.1), PV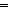 O triphyrin(2.1.1) was highly distorted and adopted a domed structure with P(V) placed at a distance of 0.927 Å above the mean plane defined by the four meso-carbon atoms. NMR studies indicated the paratropic ring current effect in the PV
O triphyrin(2.1.1) was highly distorted and adopted a domed structure with P(V) placed at a distance of 0.927 Å above the mean plane defined by the four meso-carbon atoms. NMR studies indicated the paratropic ring current effect in the PV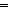 O triphyrin(2.1.1) complexes and the outer β-pyrrole protons which appear in the downfield region of 7.50–8.20 ppm in free base triphyrin(2.1.1) experienced significant upfield shifts and appeared in the region of 3.50–4.50 ppm in PV
O triphyrin(2.1.1) complexes and the outer β-pyrrole protons which appear in the downfield region of 7.50–8.20 ppm in free base triphyrin(2.1.1) experienced significant upfield shifts and appeared in the region of 3.50–4.50 ppm in PV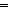 O triphyrin(2.1.1) complexes, supporting the switching of aromatic 14π free base triphyrin(2.1.1) to antiaromatic 16π PV
O triphyrin(2.1.1) complexes, supporting the switching of aromatic 14π free base triphyrin(2.1.1) to antiaromatic 16π PV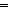 O triphyrin(2.1.1) complexes. The PV
O triphyrin(2.1.1) complexes. The PV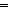 O triphyrin(2.1.1) complexes show one intense absorption band in the 300–350 nm region and an antiaromatic characteristic broad band in the lower energy region of 450–1000 nm. The electrochemical studies revealed that the PV
O triphyrin(2.1.1) complexes show one intense absorption band in the 300–350 nm region and an antiaromatic characteristic broad band in the lower energy region of 450–1000 nm. The electrochemical studies revealed that the PV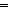 O triphyrin(2.1.1) complexes undergo very easy oxidations and difficult reductions compared to their corresponding free base triphyrins(2.1.1). DFT/TD-DFT calculations were carried out to support the experimental observations.
O triphyrin(2.1.1) complexes undergo very easy oxidations and difficult reductions compared to their corresponding free base triphyrins(2.1.1). DFT/TD-DFT calculations were carried out to support the experimental observations.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


