Urchin-like nano lawn of cobalt-iron/nitrogen-carbon activating peroxymonosulfate forced by ultrasonic action: Selective oxidation from pollutant structure
IF 8.7
Q1 Environmental Science
引用次数: 0
Abstract
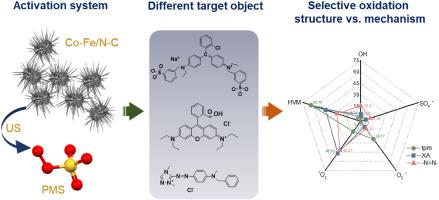
Selective oxidation in heterogeneous activation of persulfate has attracted much interest, however, the current research focuses more on the active species and less on the pollutant structure itself. In this study, the novel composite-materials, Co-Fe/N-C nanowires (CFNC-Nws), the C/N combined Co-Fe bimetallic carbonate hydroxide nano-lawn, were synthesized as activators of peroxymonosulfate (PMS). Subsequently, a representative oxidation system, CFNC-Nws (0.02 g L−1) activating PMS under ultrasonic (US, 28 kHz) mechanochemical action (the surface mechanochemical roles of dilatational wave-US driving the CFNC-Nws accompanied by particle motions), was constructed for exploring the effects of removing the different structures of pollutants including triphenylmethane-, azo-, and xanthene-compounds. It was found that without additional pH adjustment required, the coupling US/CFNC-Nws/PMS system itself could generate hydroxyl radical (•OH), sulfate radicals (SO4•−), superoxide radical (O2•−), singlet oxygen (1O2), and high-valent-metal oxidizing species (HVM) in the absence of pollutants. However, upon introducing model pollutants, although over 90% removal of them can be obtained within 15–30 min apparently, the kinds of dominant active species and their contributions for the removals of the above pollutants were widely varied, which indicated the selective oxidation from the pollutant structure. It was because of that the variations in the affinity of pollutant structures for different active species resulted in distinct removal processes and pathways. Our study provided novel insights that could be served as a foundation for selecting, designing, and implementing appropriate advanced oxidation technologies at the pollutant structure level to enhance their practical effectiveness in water treatment.
超声强制钴-铁/氮-碳活化过氧单硫酸盐的海胆状纳米草坪:污染物结构的选择性氧化
过硫酸盐非均相活化中的选择性氧化引起了人们的广泛关注,但目前的研究主要集中在活性物质上,而对污染物结构本身的研究较少。本文合成了一种新型复合材料——Co-Fe/N-C纳米线(CFNC-Nws),即C/N复合Co-Fe双金属碳酸盐氢氧化物纳米草坪,作为过氧单硫酸盐(PMS)的活化剂。随后,构建了具有代表性的氧化体系,CFNC-Nws (0.02 g L−1)在超声(US, 28 kHz)机械化学作用下激活PMS(膨胀波US驱动CFNC-Nws伴随粒子运动的表面机械化学作用),以探索去除不同结构污染物的效果,包括三苯甲烷-,偶氮-和杂蒽-化合物。结果表明,不需要额外的pH调节,耦合US/CFNC-Nws/PMS体系本身在没有污染物的情况下可以产生羟基自由基(•OH)、硫酸盐自由基(SO4•−)、超氧自由基(O2•−)、单重态氧(1O2)和高价金属氧化物质(HVM)。然而,引入模型污染物后,虽然在15 ~ 30 min内明显可以达到90%以上的去除率,但优势活性物种的种类及其对上述污染物去除率的贡献却各不相同,这表明污染物结构上存在选择性氧化。这是因为污染物结构对不同活性物种的亲和力不同,导致了不同的去除过程和途径。我们的研究提供了新的见解,可以为在污染物结构水平上选择、设计和实施适当的高级氧化技术提供基础,以提高其在水处理中的实际效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Cycle
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
20
审稿时长
45 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


