Enhancing nano zero-valent iron (nZVI) performance for Cr(VI) removal through zeolite imidazole framework-8 (ZIF-8) coating
IF 8.7
Q1 Environmental Science
引用次数: 0
Abstract
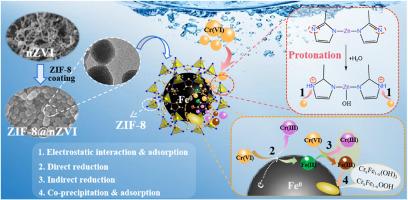
Nano zero-valent iron (nZVI) has garnered significant attention as an excellent environmental remediation material for Cr(VI) adsorption. However, its practical utilization is significantly impeded by inherent limitations, notably its propensity for aggregation and susceptibility to oxidation. This study proposed an innovative approach that using zeolite imidazole framework-8 (ZIF-8) as a cladding material for encapsulating nZVI particles. This encapsulation served to safeguard nZVI from oxidation and agglomeration. Furthermore, the ZIF-8 coating possessed suitable pore channels, allowing the migration of Cr(VI) ions towards the nZVI's surface, thereby enabling efficient reduction. The ZIF-8-coated nZVI composite (ZIF-8@nZVI) achieved a remarkable Cr(VI) removal efficiency of 98.43% under the optimized conditions. After 10 days of exposure in the aqueous solution, the efficiency remained above 84%. The uptake of Cr(VI) by ZIF-8@nZVI proceeded via a chemisorption pathway, inherently spontaneous and endothermic in character. The mechanism analysis indicated that ZIF-8 and nZVI exhibited synergistic enhancement effect on Cr(VI) removal. The protonated nitrogen groups within ZIF-8 facilitated the swift diffusion of negatively charged Cr(VI) ions to the surface of ZIF-8@nZVI. Once adsorbed, Cr(VI) was directly reduced to Cr(III) by Fe0, which in turn underwent oxidation to Fe(II)/Fe(III). Notably, Fe(II) indirectly reduced Cr(VI) to Cr(III), while itself being oxidized to Fe(III). Consequently, the resulting Cr(III) ions co-precipitated with Fe(III), forming hydroxide complexes that were eliminated from the aqueous environment. Overall, this research demonstrated the practicability of employing ZIF-8 as a coating material to develop highly efficient nZVI-based adsorbents and highlighted the potential of ZIF-8@nZVI in addressing Cr(VI) contamination challenges.
通过沸石咪唑骨架-8 (ZIF-8)涂层提高纳米零价铁(nZVI)去除Cr(VI)的性能
纳米零价铁(nZVI)作为一种吸附Cr(VI)的优良环境修复材料受到了广泛关注。然而,它的实际应用受到固有限制的严重阻碍,特别是它的聚集倾向和易氧化性。本研究提出了一种利用沸石咪唑骨架-8 (ZIF-8)作为包覆材料包封nZVI颗粒的创新方法。这种包封有助于保护nZVI免受氧化和团聚。此外,ZIF-8涂层具有合适的孔隙通道,允许Cr(VI)离子向nZVI表面迁移,从而实现高效还原。在优化条件下,zif -8包覆的nZVI复合材料(ZIF-8@nZVI)的Cr(VI)去除率达到了98.43%。在水溶液中暴露10天后,效率仍保持在84%以上。ZIF-8@nZVI对Cr(VI)的吸收是通过化学吸附途径进行的,本质上是自发的和吸热的。机理分析表明,ZIF-8与nZVI对Cr(VI)的去除具有协同增强作用。ZIF-8中的质子化氮基团促进了带负电荷的Cr(VI)离子迅速扩散到ZIF-8@nZVI表面。Cr(VI)吸附后,被Fe0直接还原为Cr(III),再被Fe0氧化为Fe(II)/Fe(III)。值得注意的是,Fe(II)间接将Cr(VI)还原为Cr(III),而自身被氧化为Fe(III)。因此,生成的Cr(III)离子与Fe(III)共沉淀,形成氢氧化物,从水环境中消除。总的来说,这项研究证明了使用ZIF-8作为涂层材料来开发高效的nzvi基吸附剂的可行性,并强调了ZIF-8@nZVI在解决Cr(VI)污染挑战方面的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Cycle
Engineering-Engineering (miscellaneous)
CiteScore
9.20
自引率
0.00%
发文量
20
审稿时长
45 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


