Highly potent quinoxalinediones inhibit α-hemolysin and ameliorate Staphylococcus aureus lung infections
IF 18.7
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
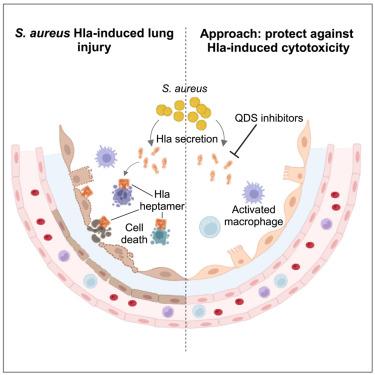
Hospital-acquired pneumonia caused by Staphylococcus aureus is associated with patient morbidity and mortality, despite adequate antibiotic therapy. This illustrates the need for treatments beyond antibiotics. The pore-forming heptameric toxin α-hemolysin (Hla) is a major pathogenicity factor of S. aureus and a clinically validated target. We identify quinoxalinediones (QDS) as highly potent Hla inhibitors, conferring protection against the hallmarks of Hla-induced pathogenicity such as Ca2+ influx, cytotoxicity, hemolysis, and monolayer destruction. The effects were exerted across major Hla subtypes in all relevant cell types. QDS prevented the formation of functional pores by interacting with Hla near the phospholipid-binding site. The QDS analog, H052, was active in mouse models of S. aureus lung infections, when administered prophylactically or therapeutically, either as monotherapy or when given in combination with the antibiotic linezolid. The study provides evidence that complex bacterial toxins can be targeted in vivo by drug-like small molecules.
高效喹啉二酮抑制α-溶血素和改善金黄色葡萄球菌肺部感染
由金黄色葡萄球菌引起的医院获得性肺炎与患者发病率和死亡率相关,尽管有适当的抗生素治疗。这说明需要抗生素以外的治疗方法。成孔七聚体毒素α-溶血素(Hla)是金黄色葡萄球菌的主要致病因子,也是临床证实的靶点。我们发现喹草胺二酮(QDS)是一种高效的Hla抑制剂,可以防止Hla诱导的致病性,如Ca2+内流、细胞毒性、溶血和单层破坏。在所有相关细胞类型的主要Hla亚型中都发挥了作用。QDS通过与磷脂结合位点附近的Hla相互作用阻止功能性孔的形成。QDS类似物H052在金黄色葡萄球菌肺部感染的小鼠模型中,无论是预防还是治疗,无论是单独治疗还是与抗生素利奈唑胺联合使用,都具有活性。这项研究提供了证据,证明复杂的细菌毒素在体内可以被类似药物的小分子靶向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


