From Bones to Bugs: Structure-Based Development of Raloxifene-Derived Pathoblockers That Inhibit Pyocyanin Production in Pseudomonas aeruginosa
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
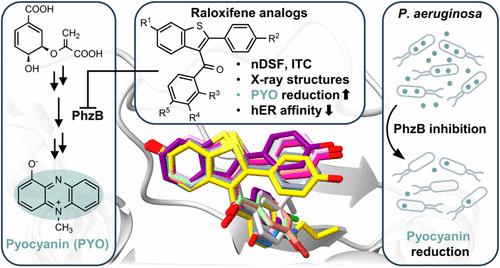
The human pathogen Pseudomonas aeruginosa is particularly notorious for its multiple resistance mechanisms. A new concept for anti-infectives is the “pathoblocker” approach, which targets virulence factors to disarm rather than kill pathogens and thus attenuates the development of resistance. Based on the estrogen receptor modulator raloxifene, which had previously been identified as a potential biosynthesis inhibitor of the virulence factor pyocyanin via in silico screening, analogues have been developed as pathoblockers against P. aeruginosa. These compounds reduce the production of pyocyanin by binding to the phenazine biosynthesis enzyme PhzB. Structure–activity relationships (SAR) were explored using nano differential scanning fluorimetry, isothermal titration calorimetry, and 12 X-ray cocrystal structures. Compared to raloxifene, congener 20c shows a 60-fold lower affinity for the human estrogen receptor with a 15-fold increase in pyocyanin inhibitory activity. The comprehensive structural information gathered in this study paves the way for the development of improved pathoblockers with increased potency and selectivity.
从骨骼到细菌:基于结构的雷洛昔芬衍生的抑制铜绿假单胞菌Pyocyanin产生的病原体阻滞剂的开发
人类病原体铜绿假单胞菌尤其因其多重抗药性机制而臭名昭著。抗感染药物的一个新概念是 "病理阻断剂 "方法,它以毒力因子为目标,解除而不是杀死病原体,从而减少抗药性的产生。雌激素受体调节剂雷洛昔芬以前曾通过硅学筛选被确定为毒力因子焦花青素的潜在生物合成抑制剂,在此基础上,我们开发了类似物作为抗铜绿假单胞菌的病理阻断剂。这些化合物通过与酚嗪生物合成酶 PhzB 结合,减少了焦花青素的产生。研究人员利用纳米差示扫描荧光测定法、等温滴定量热法和 12 种 X 射线共晶体结构探索了这些化合物的结构-活性关系(SAR)。与雷洛昔芬相比,同系物 20c 对人类雌激素受体的亲和力降低了 60 倍,但其焦花青素抑制活性提高了 15 倍。本研究收集的全面结构信息为开发具有更强效力和选择性的改良病理阻断剂铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


