Organically Functionalized Mesoporous Silica Network for One-Pot Synthesis of 5-Hydroxymethylfurfural from Glucose in Water
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The conversion of lignocellulosic biomass to 5-hydroxymethylfurfural (HMF), a key renewable molecule and a potential alternative to petroleum-based chemicals, is of great research interest. In this study, we prepared a t-SiO2@B@A catalyst consisting of a mesoporous silica support, where surface −OH groups were grafted with 3-(aminopropyl)triethoxysilane (APTES), followed by functionalization with 4-formylbenzoic acid. The synthesized t-SiO2@B@A catalyst, bearing Brønsted basic (─C═N─) and acidic (−COOH) sites, exhibited bifunctional activity for the selective production of HMF from glucose in water. The structural and chemical properties of the t-SiO2@B@A catalyst were determined using various characterization techniques, such as XRD, BET, FESEM, TGA, FTIR, TPD-NH3/CO2, and solid-state cross-polarization magic angle spinning nuclear magnetic resonance (CP MAS NMR) spectroscopy, which confirmed the successful incorporation of organic moieties onto the silica support. The mesoporosity of the silica support was well maintained after surface modification, exhibiting a surface area of 266.4 m2/g, with a total acidity of 2.27 mmol/g and thermal stability up to 400 °C. The reaction system was optimized with various parameters, such as temperature, reaction time, catalyst amount, and solvents. Consequently, an HMF yield of 69.7% was achieved after 6 h at 120 °C over the t-SiO2@B@A (0.5 w/v %) catalyst. Moreover, the catalyst showed good recyclability for eight test cycles without significant loss of catalytic activity. A kinetic model was developed to monitor the conversion of glucose and the temperature dependence of HMF production. Based on numerical modeling, the first step, isomerization of glucose to fructose, was found to be slower (rate constant: kB1 = 0.348 min–1) while the second step, dehydration of fructose to HMF is faster (rate constant: kB1 = 0.348 min–1). This study provides an efficient and environmentally benign method for the conversion of glucose to HMF, highlighting its potential for industrial applications.
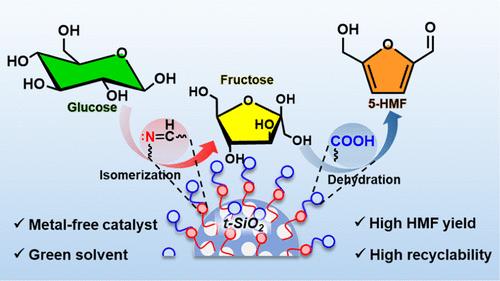
有机功能化介孔二氧化硅网络用于葡萄糖在水中一锅法合成5-羟甲基糠醛
木质纤维素生物质转化为5-羟甲基糠醛(HMF)是一种重要的可再生分子,也是石油基化学品的潜在替代品,具有很大的研究价值。在这项研究中,我们制备了一种t-SiO2@B@A催化剂,由介孔二氧化硅载体组成,其中表面- OH基团接枝3-(氨基丙基)三乙氧基硅烷(APTES),然后与4-甲酰苯甲酸官能化。合成的t-SiO2@B@A催化剂,具有Brønsted碱性(─C = N─)和酸性(−COOH)位点,表现出在水中葡萄糖选择性生产HMF的双功能活性。利用XRD、BET、FESEM、TGA、FTIR、TPD-NH3/CO2和固态交叉极化魔角自旋核磁共振(CP MAS NMR)等表征技术对t-SiO2@B@A催化剂的结构和化学性质进行了表征,证实了有机部分成功地掺入到二氧化硅载体上。表面改性后,二氧化硅载体的介孔率保持良好,比表面积为266.4 m2/g,总酸度为2.27 mmol/g,热稳定性高达400℃。通过温度、反应时间、催化剂用量、溶剂等参数对反应体系进行了优化。结果表明,在t-SiO2@B@A (0.5 w/v %)催化剂上,在120°C下反应6 h后,HMF收率达到69.7%。此外,该催化剂在8个测试循环中表现出良好的可回收性,且没有明显的催化活性损失。建立了一个动力学模型来监测葡萄糖的转化和HMF生产的温度依赖性。通过数值模拟发现,第一步葡萄糖异构化成果糖较慢(速率常数kB1 = 0.348 min-1),而第二步果糖脱水成HMF较快(速率常数kB1 = 0.348 min-1)。本研究为葡萄糖转化为HMF提供了一种高效、环保的方法,突出了其工业应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


