Photoinduced nickel-catalysed enantioconvergent sp3–sp3 coupling of unactivated olefins and aziridines
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Catalytic sp3–sp3 bond-forming reactions have been the subject of considerable interest in both academic and pharmaceutical laboratories. This is largely due to the observation that a higher content of sp3-hybridized carbons has recently been shown to improve several molecular attributes that contribute to clinical success. Although the ready availability of unactivated olefins and aziridines makes them ideal precursors to forge enantioenriched sp3–sp3 architectures with added-value amine functions, an enantioconvergent catalytic scenario of these counterparts has not yet been realized. Here we describe a nickel-catalysed blueprint that enables the enantioselective construction of amine-containing sp3–sp3 architectures via photoinduced enantioconvergent coupling of racemic aziridines with alkylzirconium reagents generated in situ from unactivated terminal and even internal olefins. The broad applicability of this protocol is illustrated in a series of late-stage diversification of advanced synthetic intermediates. Enantioconvergent sp3–sp3 coupling methods are of interest for drug development. Now a visible-light-induced Ni-catalysed ring-opening alkylation of aziridines with olefins is presented to obtain enantioenriched amine-containing sp3–sp3 architectures.
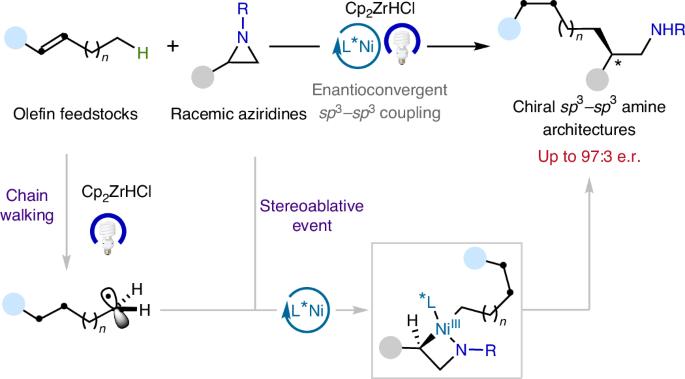

光诱导镍催化的非活化烯烃和叠氮醚的对映收敛sp3-sp3偶联
催化sp3-sp3成键反应一直是学术界和制药实验室相当感兴趣的课题。这主要是由于观察到较高含量的sp3杂化碳最近被证明可以改善一些有助于临床成功的分子属性。尽管非活化烯烃和叠氮嘧啶的现成可用性使它们成为构建具有附加价值胺功能的对映体富集sp3-sp3结构的理想前体,但这些对映体的聚合催化情景尚未实现。在这里,我们描述了一种镍催化的蓝图,通过光诱导外消旋叠氮嘧啶与未活化末端甚至内部烯烃原位生成的烷基锆试剂的对映收敛偶联,可以对含胺的sp3-sp3结构进行对映选择性构建。这一协议的广泛适用性在一系列高级合成中间体的后期多样化中得到说明。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


