Genome-wide CRISPR screen in human T cells reveals regulators of FOXP3
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
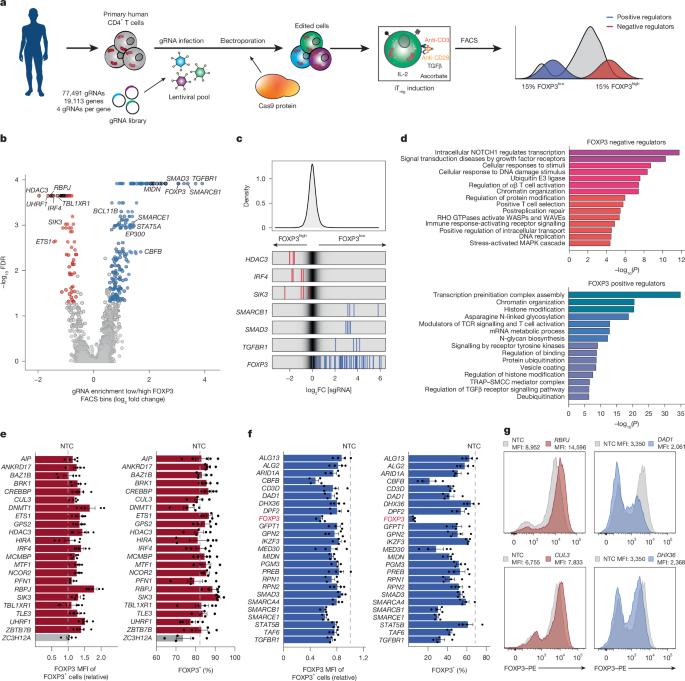
Regulatory T (Treg) cells, which specifically express the master transcription factor FOXP3, have a pivotal role in maintaining immunological tolerance and homeostasis and have the potential to revolutionize cell therapies for autoimmune diseases1–3. Although stimulation of naive CD4+ T cells in the presence of TGFβ and IL-2 can induce FOXP3+ Treg cells in vitro (iTreg cells), the resulting cells are often unstable and have thus far hampered translational efforts4–6. A systematic approach towards understanding the regulatory networks that dictate Treg differentiation could lead to more effective iTreg cell-based therapies. Here we performed a genome-wide CRISPR loss-of-function screen to catalogue gene regulatory determinants of FOXP3 induction in primary human T cells and characterized their effects at single-cell resolution using Perturb-icCITE-seq. We identify the RBPJ–NCOR repressor complex as a novel, context-specific negative regulator of FOXP3 expression. RBPJ-targeted knockout enhanced iTreg differentiation and function, independent of canonical Notch signalling. Repeated cytokine and T cell receptor signalling stimulation in vitro revealed that RBPJ-deficient iTreg cells exhibit increased phenotypic stability compared with control cells through DNA demethylation of the FOXP3 enhancer CNS2, reinforcing FOXP3 expression. Conversely, overexpression of RBPJ potently suppressed FOXP3 induction through direct modulation of FOXP3 histone acetylation by HDAC3. Finally, RBPJ-ablated human iTreg cells more effectively suppressed xenogeneic graft-versus-host disease than control iTreg cells in a humanized mouse model. Together, our findings reveal novel regulators of FOXP3 and point towards new avenues to improve the efficacy of adoptive cell therapy for autoimmune disease. The RBPJ–NCOR repressor complex is identified as a negative regulator of FOXP3 expression through modulation of histone acetylation in induced regulatory T cells.

人类T细胞全基因组CRISPR筛选揭示FOXP3调控因子
调节性T (Treg)细胞特异性表达主转录因子FOXP3,在维持免疫耐受和体内平衡中起关键作用,并有可能彻底改变自身免疫性疾病的细胞治疗方法1,2,3。虽然在TGFβ和IL-2的存在下刺激初始CD4+ T细胞可以在体外诱导FOXP3+ Treg细胞(iTreg细胞),但产生的细胞通常不稳定,迄今为止阻碍了翻译工作4,5,6。了解Treg分化调控网络的系统方法可能会导致更有效的基于Treg细胞的治疗。在这里,我们进行了全基因组的CRISPR功能缺失筛选,编目了原代人T细胞中FOXP3诱导的基因调控决定因素,并使用Perturb-icCITE-seq在单细胞分辨率下表征了它们的作用。我们发现RBPJ-NCOR抑制复合物是FOXP3表达的一种新的、环境特异性的负调控因子。靶向rbpj的敲除增强了iTreg的分化和功能,独立于典型的Notch信号。体外反复的细胞因子和T细胞受体信号刺激表明,与对照细胞相比,rbpj缺陷的iTreg细胞通过FOXP3增强子CNS2的DNA去甲基化,增强FOXP3的表达,表现出更高的表型稳定性。相反,RBPJ的过表达通过HDAC3直接调节FOXP3组蛋白乙酰化而有效抑制FOXP3的诱导。最后,在人源化小鼠模型中,rbpj消融的人iTreg细胞比对照iTreg细胞更有效地抑制异种移植物抗宿主病。总之,我们的发现揭示了FOXP3的新调控因子,并指出了提高自身免疫性疾病过继细胞治疗疗效的新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


