Natural Ellagic Acid-Polyphenol ″Sandwich Biscuit″ Self-Assembled Solubilizing System for Formation Mechanism and Antibacterial Synergia
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Ellagic acid (EA) has limited utility due to its extremely low solubility. Inspired by the naturally high content of EA in Triphala, the research group discovered that there might be noncovalent self-assembled nanoaggregates centered on EA in natural polyphenols that could significantly improve EA’s solubility and enhance its antibacterial activity. Therefore, seven polyphenols that we found were potentially involved in EA self-assembly were separated and identified from Triphala, and 18 binary, ternary, and quaternary self-assembly systems were constructed by combining them with EA. Finally, a ternary self-assembled solubilizing system centered on ellagic acid-gallic acid-catechin (EA-GA-CA) was established. The system could improve the solubility of EA from 0.95 to 171.345 μg·mL–1, leading to a notable 180-fold increase, and the stability of EA in water was increased 3 times compared with the mixture of EA, GA, and CA, which is currently the most effective carrier-free hydrotropic solubilizing method of EA. The in vitro release rate reached about 61%, which was about 60 times higher than that of EA. Exploring the formation mechanism of the self-assembled complex revealed that EA, GA, and CA were induced by hydrogen bonding and π–π stacking to form a solubilizing structure resembling a sandwich biscuit. In addition, in vitro antibacterial experiments, biofilm clearance experiments, and infected wound healing experiments demonstrated that the EA-GA-CA complex has a better inhibitory effect on Staphylococcus aureus (S. aureus) and methicillin-resistant S. aureus (MRSA) than EA, GA, CA, benzylpenicillin potassium, and the mixture of EA, GA, and CA (MIC = 12.5 mM). The inhibition rate of the EA-GA-CA complex against S. aureus reaches 82.68%, and it can rapidly promote the healing of infected wounds caused by S. aureus within 4–6 days (the healing rate increased from 15 to 75%). This study aims to provide new ideas for EA’s natural small molecule carrier-free self-assembly solubilization and synergistic applications.
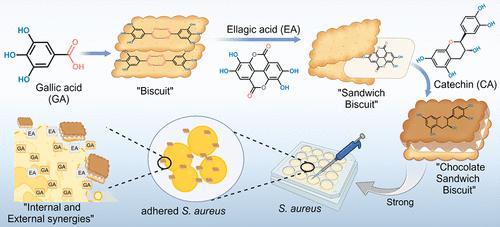
天然鞣花酸-多酚″夹心饼干″自组装增溶体系的形成机理与抗菌协同作用
鞣花酸(EA)由于其极低的溶解度而效用有限。受Triphala天然高含量EA的启发,课题组发现天然多酚中可能存在以EA为中心的非共价自组装纳米聚集体,可以显著提高EA的溶解度,增强其抗菌活性。因此,我们从Triphala中分离鉴定了7种可能参与EA自组装的多酚,并将它们与EA结合构建了18个二元、三元和四元自组装体系。最后,建立了以鞣花酸-没食子酸-儿茶素(EA- ga -ca)为中心的三元自组装增溶体系。系统可以提高溶解度的EA从0.95到171.345μg·mL-1导致显著增加180倍,和EA在水中的稳定性增加3倍与EA的混合物相比,GA, CA,目前最有效的无载体向水性的EA的增溶的方法。体外释放率达到约61%,这是60倍的EA。探索自我组装的形成机制复杂的显示,EA, GA,通过氢键和π -π堆积诱导形成类似夹心饼干的增溶结构。此外,体外抗菌实验、生物膜清除实验和感染创面愈合实验均表明,EA-GA-CA复合物对金黄色葡萄球菌(S. aureus)和耐甲氧西林金黄色葡萄球菌(MRSA)的抑制作用优于EA、GA、CA、苄西林钾以及EA、GA、CA的混合物(MIC = 12.5 mM)。EA-GA-CA复合物对金黄色葡萄球菌的抑制率达82.68%,能在4 ~ 6天内迅速促进金黄色葡萄球菌引起的感染伤口愈合(愈合率由15%提高到75%)。本研究旨在为EA天然小分子无载体自组装增溶及协同应用提供新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


