Target DNA-induced filament formation and nuclease activation of SPARDA complex
IF 25.9
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
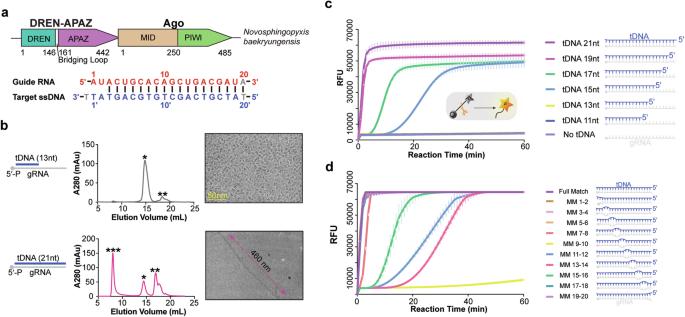
The short Argonaute-based bacterial defense system, SPARDA (Short Prokaryotic Argonaute and DNase/RNase-APAZ), utilizes guide RNA to target invading complementary DNA and exhibits collateral nuclease activity, leading to cell death or dormancy. However, its detailed mechanisms remain poorly understood. In this study, we investigated the SPARDA system from Novosphingopyxis baekryungensis (NbaSPARDA) and discovered an unexpected filament configuration upon target DNA binding, which strongly correlated with collateral nuclease activity. Filament formation and nuclease activation require a guide–target heteroduplex of sufficient length with perfect complementarity at the central region. A series of cryo-EM structures of NbaSPARDA complexes, loaded with guide RNA, target DNA of varying lengths, and substrate ssDNA, were determined at ~3.0 Å resolution. Structural analyses indicated that guide RNA binding induces dimerization of the NbaSPARDA complex, while target DNA engagement disrupts this dimerization. Further propagation of the guide–target heteroduplex triggers filament formation through a checkpoint mechanism. The NbaSPARDA filament consists of a backbone formed by interlocking short Argonaute proteins, with an inner layer composed of DREN nuclease domains. Filament formation leads to tetramerization of the monomeric DREN nuclease domain, activating its collateral nuclease activity against environmental nucleic acids — a feature leveraged for molecular diagnostics. For bacteria heterologously expressing the NbaSPARDA system, defense against invading bacteriophages and plasmids relies on filament formation. Collectively, these findings illustrate the detailed working mechanism of the NbaSPARDA complex and highlight the importance of its filament formation in host defense.

靶dna诱导丝的形成和SPARDA复合体的核酸酶激活
基于短Argonaute的细菌防御系统SPARDA (short Prokaryotic Argonaute and DNase/RNase-APAZ)利用引导RNA靶向入侵的互补DNA,并表现出附带的核酸酶活性,导致细胞死亡或休眠。然而,其详细机制仍然知之甚少。在这项研究中,我们研究了Novosphingopyxis baekryungensis (NbaSPARDA)的SPARDA系统,并发现了一个意想不到的丝结构,在目标DNA结合时,它与侧支核酸酶活性密切相关。丝的形成和核酸酶的激活需要一个长度足够且在中心区域具有完美互补的引导靶异双工。NbaSPARDA复合物的一系列低温电镜结构,装载了引导RNA,不同长度的靶DNA和底物ssDNA,在~3.0 Å分辨率下测定。结构分析表明,引导RNA结合诱导NbaSPARDA复合体的二聚化,而靶DNA结合破坏这种二聚化。导靶异质双工的进一步传播通过检查点机制触发细丝的形成。NbaSPARDA丝由一个由连锁短Argonaute蛋白组成的骨架组成,内层由DREN核酸酶结构域组成。细丝的形成导致单体DREN核酸酶结构域的四聚化,激活其对环境核酸的附带核酸酶活性-这是分子诊断的一个特征。对于异源表达NbaSPARDA系统的细菌,防御入侵的噬菌体和质粒依赖于丝的形成。总的来说,这些发现说明了NbaSPARDA复合体的详细工作机制,并强调了其丝形成在宿主防御中的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


