Development of Tricyclic 4,5-Dihydro-3H-pyrrolo[2,3-c]quinolin-4-ones as Potent Autotaxin Inhibitors for Pulmonary Fibrosis Treatment In Vivo
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Autotaxin (ATX) has been recognized as an attractive target due to its hyperactivity in hydrolyzing lysophosphatidylcholine (LPC) into lysophosphatidic acid (LPA) throughout the initiation and progression of fibrotic diseases. Herein, a hydrophilic amide linker and sp3-rich bicyclic 4,5,6,7-tetrahydro-7H-pyrazolo[3,4-c]pyridin-7-one scaffold were employed to modify the lead compound PAT-409, followed by benzene ring fusion to generate novel tricyclic 4,5-dihydro-3H-pyrrolo[2,3-c]quinolin-4-one ATX inhibitors. Among them, the pyridine-2-carboxylic derivatives 45 and 46 demonstrated subnanomolar ATX inhibition (IC50 < 1 nM), with a favorable heart safety profile (hERG > 30 μM) and minimal fibroblast toxicity. Significantly, in bleomycin-induced pulmonary fibrosis mouse models, both compounds markedly improved respiratory function and reduced fibrotic lesions. Mechanistic studies revealed that 45 suppressed the TGF-β/Smad signaling pathway, downregulating α-smooth muscle actin (α-SMA) and extracellular matrix components (ECM). Overall, the identification of 45 and 46 for pulmonary fibrosis therapy provides a featured tricyclic scaffold for further development of novel ATX inhibitors.
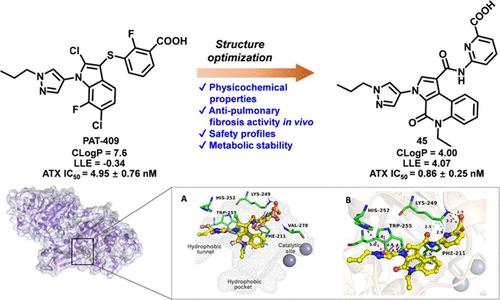
三环4,5-二氢- 3h -吡咯[2,3-c]喹啉-4-酮在体内治疗肺纤维化的有效自taxin抑制剂的研究进展
Autotaxin (ATX)被认为是一个有吸引力的靶标,因为它在纤维化疾病的发生和发展过程中具有将溶血磷脂酰胆碱(LPC)水解成溶血磷脂酸(LPA)的高活性。本文采用亲水性酰胺连接剂和富含sp3的双环4,5,6,7-四氢- 7h -pyrazolo[3,4-c]吡啶-7- 1支架对先导化合物PAT-409进行修饰,然后通过苯环融合生成新的三环4,5-二氢- 3h -pyrrolo[2,3-c]喹啉-4- 1 ATX抑制剂。其中,吡啶-2-羧基衍生物45和46表现出亚纳摩尔的ATX抑制作用(IC50 <;1 nM),具有良好的心脏安全性(hERG >;30 μM)和最小的成纤维细胞毒性。值得注意的是,在博莱霉素诱导的肺纤维化小鼠模型中,这两种化合物都能显著改善呼吸功能并减少纤维化病变。机制研究表明,45抑制TGF-β/Smad信号通路,下调α-平滑肌肌动蛋白(α-SMA)和细胞外基质成分(ECM)。总之,鉴定出45和46用于肺纤维化治疗为进一步开发新型ATX抑制剂提供了一个有特色的三环支架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


