Intrapulmonary-administered myeloid derived suppressor cells rescue mice from Pseudomonas aeruginosa infection and promote a regulatory/repair phenotype
IF 7.6
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Pseudomonas aeruginosa (P.aeruginosa) is a pathogenic opportunistic bacterium, classified as a priority by the WHO for the research of new treatments. As this bacterium is harmful through the inflammation and tissue damage it causes, we investigated the role of Myeloid Derived Suppressor Cells (MDSC) in P.aeruginosa infections and their potential as a therapeutic tool. Using both ‘classically’ obtained MDSC (through mice bone-marrow differentiation), and a new procedure developed here (using the ER-Hoxb8 hematopoietic cell line), we observed that after administering intra-nasally a lethal dose of P.aeruginosa (PAO1), intra-pulmonary transfer of MDSC, in both prophylactic and therapeutic protocols, markedly improves survival of P.aeruginosa infected animals. Mechanistically, with a sub-lethal dose of P.aeruginosa, we observed that MDSC transfer modulated lung tissue injury, down-regulated inflammatory responses and elicited lung repair. We further showed that WT-PAO1 and MDSC (and their subtypes PMN-MDSC and M−MDSC) could interact directly in vitro and in vivo, and that both PMN- and M−MDSC gene expression (assessed through RNA sequencing) was modulated after in vitro P.aeruginosa infection, and that WT-PAO1 (but not ΔFlic-PAO1) infection led to inhibition of T cell proliferation and promoted epithelial cell wound healing. Furthermore, we showed that the transcription factor Nr4A1 was up-regulated in both PMN- and M−MDSC- infected cells and may be an important mediator in the process. Altogether, we highlight a potential beneficial role of MDSC in P.aeruginosa infection responses and suggest that the unique properties of these cells make them attractive potential new therapeutic tools for patients with acute or chronic inflammatory diseases.
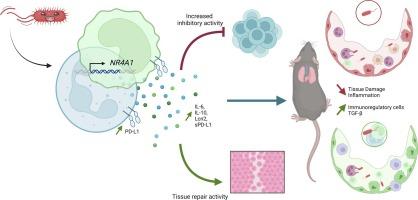
肺内注射骨髓源性抑制细胞可使小鼠免于铜绿假单胞菌感染,并促进调节/修复表型。
铜绿假单胞菌(P.aeruginosa)是一种致病性机会性细菌,被世界卫生组织列为研究新疗法的重点。由于这种细菌通过引起炎症和组织损伤而有害,我们研究了髓系衍生抑制细胞(MDSC)在铜绿假单胞菌感染中的作用及其作为治疗工具的潜力。使用“经典”获得的MDSC(通过小鼠骨髓分化)和这里开发的新程序(使用ER-Hoxb8造血细胞系),我们观察到,在鼻内给予致死剂量的铜绿假单胞菌(PAO1)后,肺内MDSC转移,在预防和治疗方案中,显着提高了铜绿假单胞菌感染动物的存活率。在机制上,在亚致死剂量的p.e uluginosa下,我们观察到MDSC转移调节肺组织损伤,下调炎症反应并引发肺修复。我们进一步发现WT-PAO1和MDSC(及其亚型PMN-MDSC和M-MCSF)可以在体外和体内直接相互作用,PMN-和M-MDSC基因表达(通过RNA测序评估)在体外铜绿假单胞菌感染后被调节,WT-PAO1(而不是ΔFlic-PAO1)感染导致T细胞增殖抑制,促进上皮细胞伤口愈合。此外,我们发现转录因子Nr4A1在PMN和M-MDSC感染细胞中均上调,可能是这一过程中的重要中介。总之,我们强调了MDSC在铜绿假单胞菌感染反应中的潜在有益作用,并建议MDSC的独特性质使其成为急性或慢性炎症性疾病患者有吸引力的潜在新治疗工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mucosal Immunology
医学-免疫学
CiteScore
16.60
自引率
3.80%
发文量
100
审稿时长
12 days
期刊介绍:
Mucosal Immunology, the official publication of the Society of Mucosal Immunology (SMI), serves as a forum for both basic and clinical scientists to discuss immunity and inflammation involving mucosal tissues. It covers gastrointestinal, pulmonary, nasopharyngeal, oral, ocular, and genitourinary immunology through original research articles, scholarly reviews, commentaries, editorials, and letters. The journal gives equal consideration to basic, translational, and clinical studies and also serves as a primary communication channel for the SMI governing board and its members, featuring society news, meeting announcements, policy discussions, and job/training opportunities advertisements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


