Water structure and electric fields at the interface of oil droplets
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Interfacial water exhibits rich and complex behaviour1, playing an important part in chemistry, biology, geology and engineering. However, there is still much debate on the fundamental properties of water at hydrophobic interfaces, such as orientational ordering, the concentration of hydronium and hydroxide, improper hydrogen bonds and the presence of large electric fields2–5. This controversy arises from the challenges in measuring interfacial systems, even with the most advanced experimental techniques and theoretical approaches available. Here we report on an in-solution, interface-selective Raman spectroscopy method using multivariate curve resolution6,7 to probe hexadecane-in-water emulsions, aided by a monomer-field theoretical model for Raman spectroscopy8. Our results indicate that oil–water emulsion interfaces can exhibit reduced tetrahedral order and weaker hydrogen bonding, along with a substantial population of free hydroxyl groups that experience about 95 cm−1 redshift in their stretching mode compared with planar oil–water interfaces. Given the known electrostatic zeta potential characteristic of oil droplets9, we propose the existence of a strong electric field (about 50–90 MV cm−1) emanating from the oil phase. This field is inferred indirectly but supported by control experiments and theoretical estimates. These observations are either absent or opposite in the molecular hydrophobic interface formed by small solutes or at planar oil–water interfaces. Instead, water structural disorder and enhanced electric fields emerge as unique features of the mesoscale interface in oil–water emulsions, potentially contributing to the accelerated chemical reactivity observed at hydrophobic–water interfaces10–13. Raman spectroscopy measurements of water at the interface with oil droplets show a perturbed hydrogen-bond network and evidence for a strong interfacial electric field.
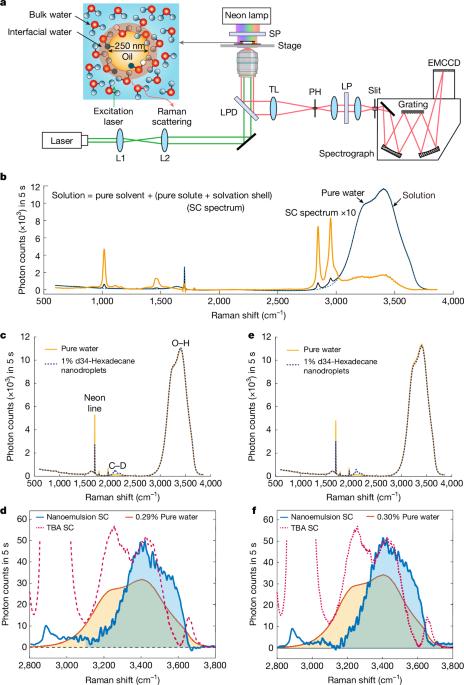

油滴界面处的水结构和电场
界面水表现出丰富而复杂的行为,在化学、生物学、地质学和工程学中发挥着重要作用。然而,关于疏水界面上的水的基本性质,如取向顺序、水合氢离子和氢氧根的浓度、不适当的氢键和大电场的存在,仍然存在许多争论。即使使用最先进的实验技术和理论方法,这种争议也源于测量界面系统的挑战。在这里,我们报告了一种溶液中,界面选择性拉曼光谱方法,使用多元曲线分辨率6,7来探测水中十六烷乳液,并借助于拉曼光谱的单体场理论模型8。我们的研究结果表明,与平面油水界面相比,油水乳液界面可以表现出降低的四面体顺序和较弱的氢键,以及大量的自由羟基,在其拉伸模式下经历约95 cm−1的红移。考虑到已知油滴的静电zeta电位特性,我们提出存在一个从油相发出的强电场(约50-90 MV cm−1)。这个领域是间接推断出来的,但有控制实验和理论估计的支持。在由小溶质形成的分子疏水界面或平面油水界面中,这些观察结果要么不存在,要么相反。相反,水结构紊乱和增强的电场是油水乳液中尺度界面的独特特征,可能有助于在疏水界面上观察到的加速化学反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


