Synthesis of Mono-Boc-2,5-Diketopiperazine: A Key Building Block for Amide and Peptide Synthesis
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
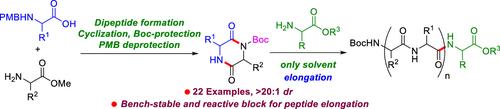
Diketopiperazine (DKP), a versatile scaffold, is extensively used in the synthesis of complex natural products, bioactive molecules, and smart materials in organic chemistry. Recently, activated DKPs, such as Boc-DKPs, have emerged as key building blocks for peptide elongation in peptide synthesis. In this study, we developed a facile protocol for synthesizing mono-Boc-protected DKPs from readily accessible N-4-methoxybenzyl (N-PMB)-amino acids and amino acid methyl esters. This protocol involved a sequence of reactions encompassing the formation of dipeptides from N-PMB-amino acids and amino acid methyl esters, cyclization of N-PMB-dipeptides to form PMB-DKPs, Boc-protection of PMB-DKPs, and subsequent PMB-deprotection of PMB-DKP-Boc to afford mono-Boc-DKPs. The protocol demonstrated a broad substrate scope, accommodating diverse amino acids with various side chains, affording mono-Boc-DKPs in good yields with excellent stereoselectivities (>20:1 dr). The synthetic utility of mono-Boc-DKPs was showcased in peptide synthesis by synthesizing pentapeptide Boc-l-Tyr(t-Bu)-Gly-l-Phe-Gly-l-Val-OtBu by 2-fold peptide elongation with two mono-Boc-DKPs. Furthermore, we synthesized Leu-enkephalin pentapeptide by reacting cyclo(Boc-l-Tyr(t-Bu)-Gly-) with H-Gly-l-Phe-l-Leu-Ot-Bu, resulting in a good yield and excellent optical purity.
单boc -2,5-二酮哌嗪的合成:酰胺和肽合成的关键组成部分
双酮哌嗪(DKP)是一种多功能支架,广泛用于有机化学中复杂天然产物、生物活性分子和智能材料的合成。最近,活化的DKPs,如Boc-DKPs,已成为肽合成中肽延伸的关键构建块。在这项研究中,我们开发了一种简单的方案,用于从易于获取的n -4-甲氧基苄基(N-PMB)氨基酸和氨基酸甲酯中合成单boc保护的DKPs。该方案涉及一系列反应,包括由n - pmb -氨基酸和氨基酸甲酯形成二肽,n - pmb -二肽环化形成PMB-DKPs, PMB-DKPs的boc保护,以及随后PMB-DKP-Boc的pmb -去保护以获得单boc - dkps。该方案显示出广泛的底物范围,可容纳具有各种侧链的多种氨基酸,提供具有良好立体选择性的高产单boc - dkps (20:1 dr)。以两个单boc - dkps为原料,经2倍肽延伸合成Boc-l-Tyr(t-Bu)- gly -l- phe - gly -l- valo - otbu五肽,证明了单boc - dkps在肽合成中的应用价值。此外,我们还通过环(Boc-l-Tyr(t-Bu)- gly -)与h - gly -l- ph -l- leu - ot - bu反应合成了leu -脑啡肽五肽,收率高,光学纯度高。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


