Efficient degradation of metronidazole by peroxodisulfate through triboelectric effect of water eddy: Electrocatalytic promotion of Fe3+/Fe2+ cycling
IF 13.2
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
In order to address the problem of permeable reactive barrier (PRB) clogging due to the generation of substantial iron sludge resulting from the release of excess Fe3+ ions from zero-valent iron in groundwater pollution control, we had introduced electrocatalytic technology to form a Fe3+/Fe2+ cycle in order to improve the clogging of PRB. On the other hand, the power source of Fe3+ reduction becomes the limiting factor for efficiency. The principle of triboelectricity has been expanded to solid–liquid frictional electrification and innovative electric energy harvesting applications. The mechanical energy converts into electrical energy under hydraulic conditions, and the generated electrons drive the Fe3+ reduction. We synthesized a contact-electro-catalyst, iron-loaded mesoporous carbon (Fe/OMC), which induced solid–liquid friction at the Fe/OMC interface under water eddy, and investigated its triboelectric response through electrochemical methods. The electrons generated by triboelectric effect form a Fe3+/Fe2+ cycle on the catalyst surface through electron transfer. Fe/OMC as a contact-electro-catalyst synergized with peroxodisulfate (PDS) to remove 91 % of metronidazole in 60 min under water eddy conditions. The system promoted the generation of free radicals by PDS and their oxidation of MNZ (>80 % for and ·OH). Cycling experiments and simulated column experiments demonstrated the cyclic regeneration performance and repair longevity of Fe/OMC increased more than 2 times compared to the theoretical life. The content of iron ions was detected and the Fe3+/Fe2+ cycling mechanism was verified by material characterization and cyclic voltammetry curves. This study contributes to the understanding of the water eddy friction triggering mechanism and provides new insights for the development of contact-electro-catalysis in the field of low-energy environment treatment.
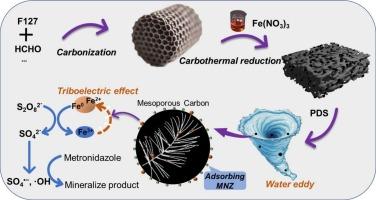
水涡流摩擦电作用下过硫酸氢盐高效降解甲硝唑:电催化促进Fe3+/Fe2+循环
为了解决地下水污染治理中零价铁释放过量Fe3+离子产生大量铁污泥导致的可渗透反应屏障(PRB)堵塞问题,我们引入电催化技术形成Fe3+/Fe2+循环,以改善PRB的堵塞。另一方面,Fe3+还原的功率来源成为效率的限制因素。摩擦电的原理已经扩展到固液摩擦电气化和创新的电能收集应用。机械能在液压条件下转化为电能,产生的电子驱动Fe3+还原。合成了负载铁的介孔碳(Fe/OMC)接触电催化剂,在水涡流作用下在Fe/OMC界面处诱导固液摩擦,并通过电化学方法研究了其摩擦电响应。摩擦电效应产生的电子通过电子转移在催化剂表面形成Fe3+/Fe2+循环。Fe/OMC作为接触电催化剂与过硫酸氢盐(PDS)协同作用,在60 min内对甲硝唑的去除率为91% %。该体系促进了PDS自由基的生成及其对MNZ的氧化(对SO4·-和·OH的氧化率>;80 %)。循环实验和模拟柱实验表明,Fe/OMC的循环再生性能和修复寿命比理论寿命提高了2倍以上。通过材料表征和循环伏安曲线验证了Fe3+/Fe2+的循环机理。本研究有助于理解水涡流摩擦触发机理,为低能耗环境处理领域接触电催化的发展提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


