Mechanistic insights into the stereocontrolling non-covalent π interactions in Pd-catalyzed redox-relay Heck arylation reaction†
IF 4.2
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The mechanism and origin of enantioselectivity of palladium-catalyzed redox-relay Heck arylation of 1,1-disubstituted homoallylic alcohols were investigated computationally. The computed mechanism consists of an initial migratory insertion, followed by a β-hydride elimination, and a subsequent re-insertion/elimination process to yield an enol intermediate, which tautomerizes to the more stable carbonyl product. Results from DFT calculations suggest that the key enantiodetermining step is the reinsertion of an alkene intermediate into the Pd–H bond, but not the initial migratory insertion of the substrate into the Pd-Aryl species. By comparing two chiral pyridine oxazoline ligands with a focus on the phenyl versus tert-butyl substituent effects, a plethora of attractive non-covalent π interactions, including CH–π interaction, lone pair–π interaction and T-shaped π–π interaction, are identified to play a key role in enabling high enantioselectivity of this reaction. This work provides mechanistic insights into the comprehensive understanding of this catalytic cascade, and highlights the significant role played by non-covalent π interactions in its enantiocontrol.
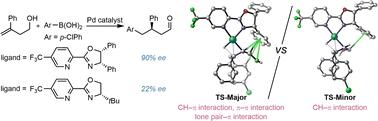
pd催化氧化还原-接力Heck芳基化反应中立体控制非共价π相互作用的机理研究。
用计算方法研究了钯催化1,1-二取代均烯丙醇氧化还原-接力Heck芳基化反应对映选择性的机理和来源。计算的机制包括最初的迁移插入,随后是β-氢化物消除,以及随后的重新插入/消除过程,以产生烯醇中间体,该中间体再异构为更稳定的羰基产物。DFT计算结果表明,关键的对映体决定步骤是将烯烃中间体重新插入Pd-H键,而不是将底物迁移插入Pd-Aryl物种。通过比较两种手性吡啶恶唑啉配体对苯基取代基和叔丁基取代基取代基的影响,发现了大量有吸引力的非共价π相互作用,包括CH-π相互作用、孤对-π相互作用和t形π-π相互作用,在该反应的高对映选择性中起着关键作用。这项工作为全面理解这种催化级联提供了机制见解,并强调了非共价π相互作用在其对映体控制中的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


