Time-Controlled Dual Targeting to Program Systemic and Intercellular Transfer of Therapeutic Effects
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Cell–cell communication serves as a foundation for intercellular therapeutic hand-over. Despite the commonsense level of understanding, no clear projection has been made to prove the mechanism. Here, the hand-over of aspirin from monocytes to inflamed cells is validated using a high-resolution time series of 3D imaging in vitro, with in vivo confirmation. Notably, caveolin is identified to play a major role in mediating the hand-over using cell receptors by super-resolution microscopy, which is induced by the overexpression of caveolin upon inflammation. When aspirin-liposomes are loaded into splenic monocytes, they naturally target inflamed sites efficiently because spleen is a major site of liposomal clearance from the body, in addition to monocyte residence to leave toward inflammatory signals. The delivery efficiency and anti-inflammatory effects of hand-over through intravenous injection are superior to those of oral injection of soluble aspirin, as confirmed in the ischemic hindlimb and fatty liver of mice (targeted therapy). These results are also agreed by the antiplatelet effect in mouse blood over 7 days (prolonged therapy) and the combination of these therapeutic actions effectively rescues the atherosclerotic carotid arteries of mice. This study demonstrates the working mechanism of the hand-over, suggesting a translational strategy to improve intercellular delivery.
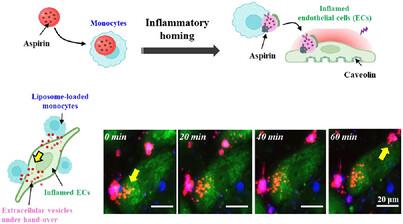
时间控制的双重靶向程序系统和细胞间转移的治疗效果
细胞间通讯是细胞间治疗交接的基础。尽管有常识性的理解,但没有明确的预测来证明这一机制。在这里,使用体外高分辨率时间序列3D成像验证了阿司匹林从单核细胞到炎症细胞的过渡,并在体内证实。值得注意的是,通过超分辨率显微镜发现,小窝蛋白在介导细胞受体的移交中发挥了主要作用,这是由炎症时小窝蛋白的过度表达引起的。当阿斯匹林脂质体被装载到脾单核细胞中时,它们自然有效地靶向炎症部位,因为脾脏是脂质体从体内清除的主要部位,除了单核细胞停留以离开炎症信号。在小鼠缺血性后肢和脂肪肝(靶向治疗)实验中证实,静脉注射移交的递送效率和抗炎作用优于口服注射可溶性阿司匹林。这些结果也与小鼠血液中7天(延长治疗)的抗血小板作用一致,这些治疗作用的联合有效地挽救了小鼠颈动脉粥样硬化。本研究证明了这种传递的工作机制,提出了一种改善细胞间传递的转化策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


