Sustainable Polyhydroxybutyrate Production via Metabolic Flux Redirection Using Clustered Regularly Interspaced Short Palindromic Repeats Interference in Escherichia coli and Carbon Capture with Microalgae
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The pursuit of biobased alternatives to petrochemical plastics has been driven by the oil crisis and climate change challenges, with polyhydroxybutyrate (PHB) emerging as a promising candidate due to its biodegradability and material properties. This study leverages metabolic engineering strategies in Escherichia coli WT7L to enhance PHB production. By employing the phaCAB operon from Caldimonas manganoxidans under a T7 promoter and implementing clustered regularly interspaced short palindromic repeats interference (CRISPRi)-mediated gene downregulation, we systematically optimized metabolic pathways. Target genes (ldhA, poxB, and pta) were selected for downregulation based on their roles in competing pathways that divert acetyl-CoA from PHB synthesis, leading to a 1.3-fold increase in PHB production, reaching 1.94 g/L. However, further inhibition of adhE, arcA, and gltA disrupted metabolic balance, reducing the PHB titer to 0.7 g/L. Integrative enhancements using GroELS on the chromosome boosted biomass and PHB titer by 3.06-fold in the optimal CRISPRi design. Further expression of phosphoenolpyruvate carboxylase (ppc) for CO2 assimilation elevated the PHB yield to 3.1 g/L. Finally, fed-batch fermentation significantly increased biomass to 17.06 g/L and PHB titer to 5.6 g/L after 72 h. Notably, released CO2 was utilized to cultivate Chlorella sorokiniana, reaching 2.26 g/L for the first time. Our results demonstrate a novel closed-loop bioprocess with the potential for net-zero emissions in sustainable biopolymer production.
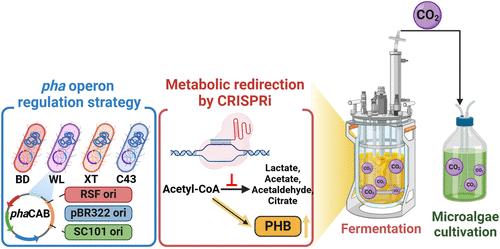
利用聚类规则间隔短回文重复序列干扰的大肠杆菌代谢通量重定向和微藻碳捕获的可持续多羟基丁酸盐生产
石油危机和气候变化的挑战推动了对生物基塑料替代品的追求,聚羟基丁酸酯(PHB)因其可生物降解性和材料性能而成为一个有希望的候选材料。本研究利用代谢工程策略在大肠杆菌WT7L中提高PHB的产量。通过在T7启动子下利用锰氧化Caldimonas manganoxidans的phaCAB操纵子,并实施集群规则间隔短回文重复序列干扰(CRISPRi)介导的基因下调,我们系统地优化了代谢途径。根据靶基因ldhA、poxB和pta在将乙酰辅酶a从PHB合成中转移的竞争途径中的作用,选择它们进行下调,导致PHB产量增加1.3倍,达到1.94 g/L。然而,进一步抑制adhE、arcA和gltA会破坏代谢平衡,使PHB滴度降至0.7 g/L。在优化的CRISPRi设计中,在染色体上使用GroELS进行整合增强,使生物量和PHB滴度提高了3.06倍。进一步表达用于CO2同化的磷酸烯醇丙酮酸羧化酶(ppc)将PHB的产量提高到3.1 g/L。最后,分批补料发酵72h后,生物量显著提高至17.06 g/L, PHB滴度显著提高至5.6 g/L,其中释放的CO2用于培养小球藻,首次达到2.26 g/L。我们的研究结果展示了一种新型闭环生物过程,具有在可持续生物聚合物生产中实现净零排放的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


