In Situ Electrochemical Activation Strategy toward Organic Cation Preintercalated Layered Vanadium-Based Oxide Cathode for High-Performance Aqueous Zinc-Ion Batteries
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
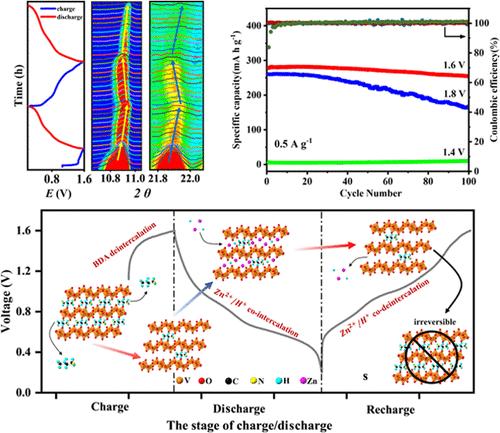
Layered vanadium-based oxides with preintercalated metal cations are attracting extensive attention as highly promising candidates for aqueous zinc-ion batteries (AZIBs) due to the increase in structural stability originating from the pillar effect. However, the strong electrostatic interaction between the rigid metal cation pillars and zinc ions results in sluggish ionic transport, thereby limiting the high-rate performance. Herein, a layered vanadium-based oxide with protonated 1,4-diaminobutane organic cation (BDA) pillars is designed as a cathode material for AZIBs. Due to the larger radius and stronger interconnection with the VO layers, the organic cation guests acting as pillars not only ensure a large interlayer space but also significantly enhance the structural stability of the layered host. Furthermore, by adopting an in situ electrochemical activation strategy, the quantitative control of the organic cation pillar content is effectively achieved. The irreversible removal of partial pillar guests not only weakens its steric buckling effects on the zinc ion but also provides more effective sites for zinc ion storage. As anticipated, the resulting (H3N(CH2)4NH3)[V6O14] (BDA-VO) electrode material exhibits an excellent electrochemical property with a high reversible specific capacity of 345 mAh g–1 at a current density of 0.1 A g–1 and an excellent cycle stability with 93.2% capacity retention over 2000 cycles at 5 A g–1.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


