Multi-element Compound-Specific Stable Isotope Analysis (2H, 13C, 15N, 33/34S) to characterize the mechanism of sulfate and hydroxyl radical reaction and photolysis of benzothiazole
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
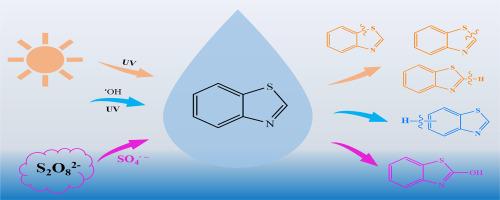
Benzothiazole was taken as a simple emerging aromatic heterocyclic contaminant as model compounds for analyzing multi-element isotope (ME-CSIA) fractionation (2H, 13C, 15N and 33/34S) for the first time, in order to obtain information on the reaction mechanism upon sulfate and hydroxyl radical reactions and photolysis. The sulfur isotope effects 33/34S to allow to explore reactions mechanisms with respect to mass dependent and independent kinetic isotope effect. For compound specific isotope analysis for 2H, 13C, and 15N using GC-pyrolysis and combustion IRMS techniques were applied and for 33/34S isotope analysis a novel approach using GC- multi collector ICPMS were developed. The multi-element fractionation factors of the radical reactions were obtained to characterize the first irreversible degradation step in order explore their potential to analyze radical oxidation processes in technical and natural systems. The hydroxyl radical reactions yield small carbon (εC = −0.67 ± 0.06‰), large hydrogen (εH = −8.8 ± 0.9‰), and negligible nitrogen and sulfur isotope fractionations as cleavage of the C−H bond the benzene ring is the first irreversible step. The heat-activated persulphate oxidation at pH = 2, dominated by SO4 •− radicals were associated with significant for C (εC = −1.56 ± 0.09‰), N (εN = 1.08 ± 0.05‰), and S (ε33S = −0.6 ± 0.04‰, ε34S = −1.1 ± 0.09‰), and negligible for H isotope fraction, indicating cleavage of the C−S bond.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


